Multiplex fluorescence PCR kit for detecting prostate cancer
A multiple fluorescence, prostate cancer technology, applied in recombinant DNA technology, microbial determination/inspection, DNA/RNA fragments, etc., can solve the problems of insufficient sensitivity, time-consuming and cumbersome, and expensive sequencing technology, and achieve sensitivity and specificity. High and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Detection of multiple fluorescent PCR primer sets and probe sets for prostate cancer
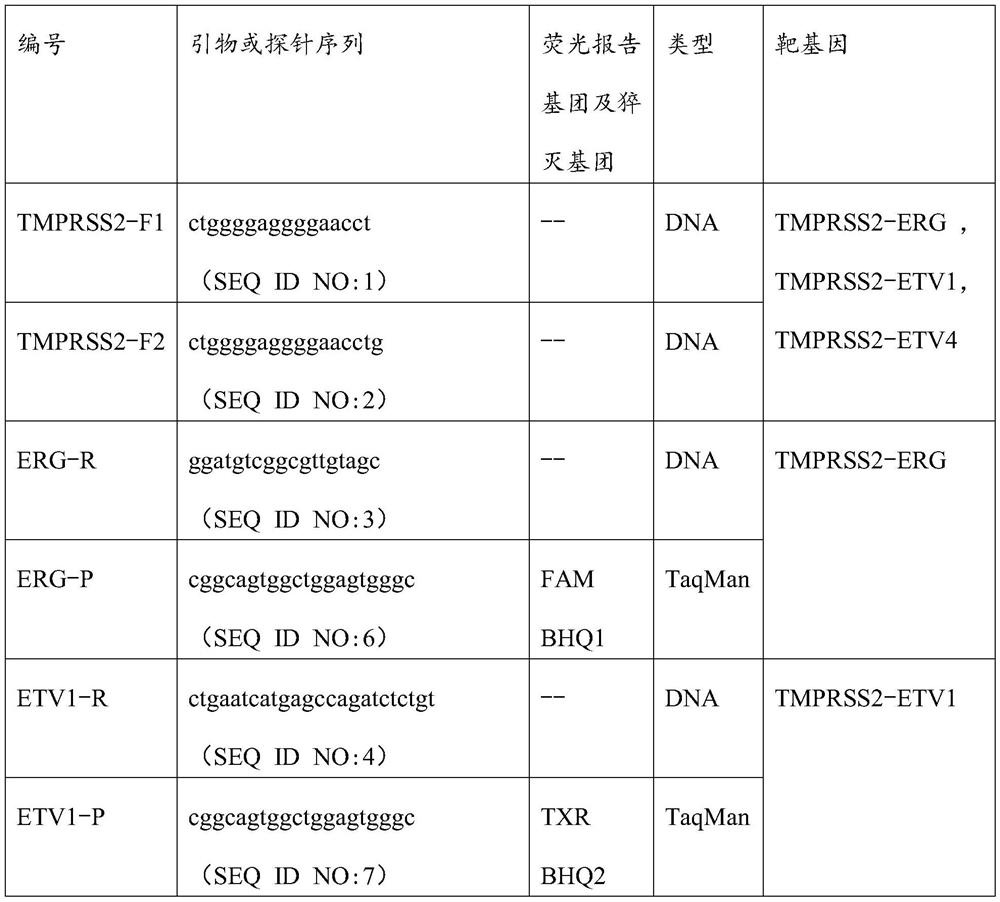
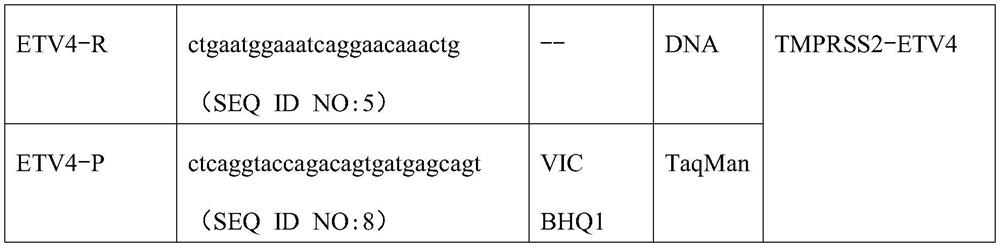
[0018] TMPRSS2-ERG, TMPRSS2-ETV1 and TMPRSS2-ETV4 are three highly specific and common fusion genes in prostate cancer, which can be used as a combination of markers to detect prostate cancer. For this marker combination, the inventors designed a multiplex fluorescent PCR primer set and probe set that can quickly detect this marker combination, the primer set includes TMPRSS2-F1, TMPRSS2-F2, ERG-R, ETV1-R and ETV4-R , its nucleotide sequence is shown in SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3, SEQ ID NO:4 and SEQ ID NO:5 respectively, TMPRSS2-ERG, TMPRSS2-ETV1 and TMPRSS2- The upstream primers of the three fusion genes of ETV4 are TMPRSS2-F1 and / or TMPRSS2-F2, and the downstream primers are ERG-R, ETV1-R and ETV4-R respectively. The specific sequences are shown in Table 1 below:
[0019] Table 1 Multiplex fluorescent PCR primer set and probe set for detection of prostate canc...
Embodiment 2
[0022] Embodiment 2 is used for detecting the multiplex fluorescent PCR kit of prostate cancer
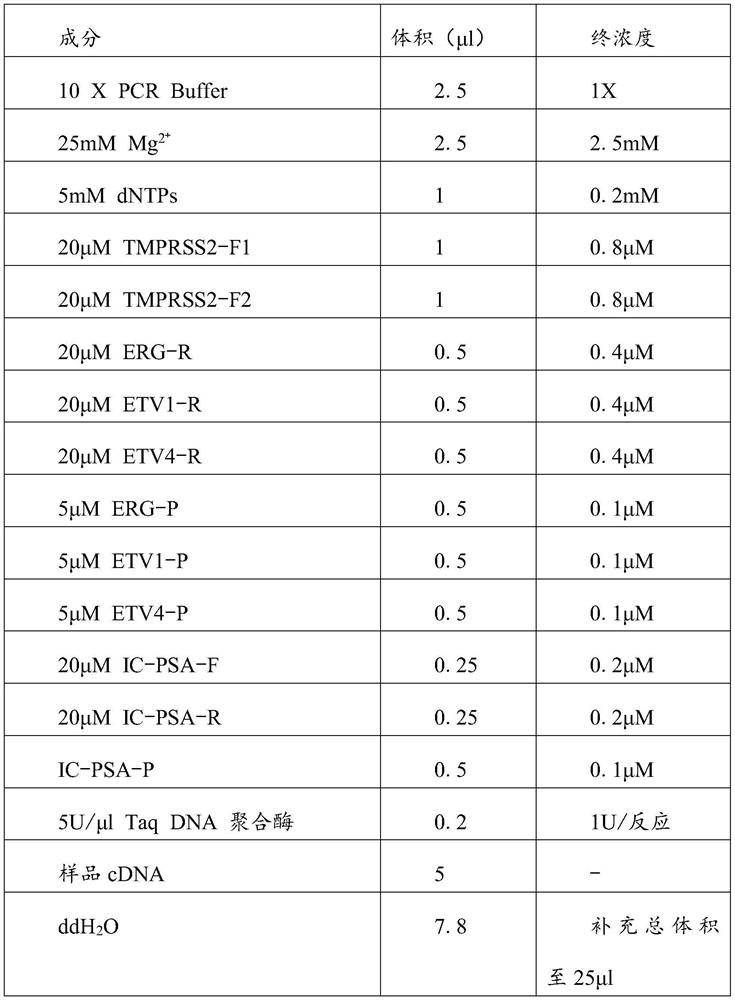
[0023] The multiplex fluorescent PCR kit for detecting prostate cancer includes the multiplex fluorescent PCR primer set and probe set for detecting prostate cancer provided in Example 1.
[0024] In a preferred embodiment, it also includes a primer pair and a fluorescent probe for detecting the internal reference gene PSA, the primer pair for the detection internal reference gene PSA is IC-PSA-F and IC-PSA-R, and its nucleotide sequences are respectively As shown in SEQ ID NO:9 and SEQ ID NO:10, the fluorescent probe for detecting the internal reference gene PSA is IC-PSA-P, and its nucleotide sequence is shown in SEQ ID NO:11.
[0025] SEQ ID NO:9cgagaagcattcccaaccc
[0026] SEQ ID NO:10tgccgacccagcaagat
[0027] SEQ ID NO:11gcccactgcatcaggaacaa
[0028] In a preferred embodiment, the 5' end of the fluorescent probe IC-PSA-P is labeled with a fluorescent reporter group Cy5, an...
Embodiment 4
[0047] Example 4 Use the kit of the invention to detect prostate cancer
[0048] In this embodiment, a total of 108 samples to be tested were obtained. According to the results of pathological biopsy, 43 cases of prostate cancer patients were diagnosed, 15 cases of benign prostatic hyperplasia patients, and 50 cases of non-cancerous normal people. Each sample was detected using the kit in Example 2 and the method in Example 3, and the results are shown in Table 3 below. The sensitivity of using the kit of this patent to detect prostate cancer was 69.7% (30 / 40), and the specificity The sex ratio was 96.9% (63 / 65), the positive predictive value was 93.8% (30 / 32), and the negative predictive value was 72.4% (63 / 87).
[0049] Table 3 Comparison of pathological biopsy results and multiple fluorescent PCR detection results of 108 samples
[0050]
[0051]
[0052] Among the 32 positive samples detected by this patent, the types of fusion genes are shown in Table 4 below. Of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com