Method and detection kit for detecting ERBB2 gene amplification based on digital PCR technology
A gene amplification and technical detection technology, applied in the method and its detection kit, is based on the digital PCR technology to detect the ERBB2 gene amplification field, which can solve the real-time and dynamic monitoring of the ERBB2 gene state of tumor cells, which is disadvantageous to wide application, To solve the problem of high patient charges, to achieve convenient auxiliary diagnosis and clinical treatment guidance, improve the success rate, and achieve good results in data amplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
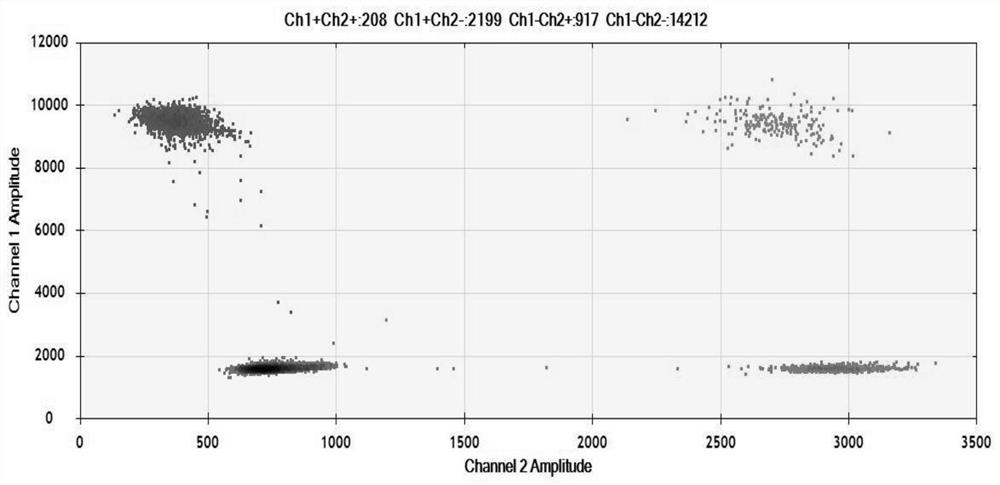
[0045] Detection of ERBB2 gene amplification in plasma samples from patients with gastric cancer by ddPCR.
[0046] 1. The steps of micro-droplet digital PCR detection are as follows:
[0047] 1) Preparation of samples: The source of sample DNA is the blood samples of patients with gastric cancer. The DNA sample can be properly diluted to ensure that the sample signal copy number is between 1×102 copy / μL and 1×105 copy / μL.
[0048]2) Prepare the PCR reaction solution according to the following ratio: 2×ddPCR Master Mix, primers, probes, 1 μL cfDNA template, make up to a final volume of 20 μL with distilled water, and configure a digital PCR mixture, in which the content of each primer is 400 nM , each probe is 500nM, and the cfDNA template is 1-10ng / μL.
[0049] 3) Load the prepared 20 μL digital PCR reaction solution onto the chip.
[0050] 4) Put the chip into the PCR machine, and carry out the amplification reaction according to the following conditions: pre-denaturation...
Embodiment 2
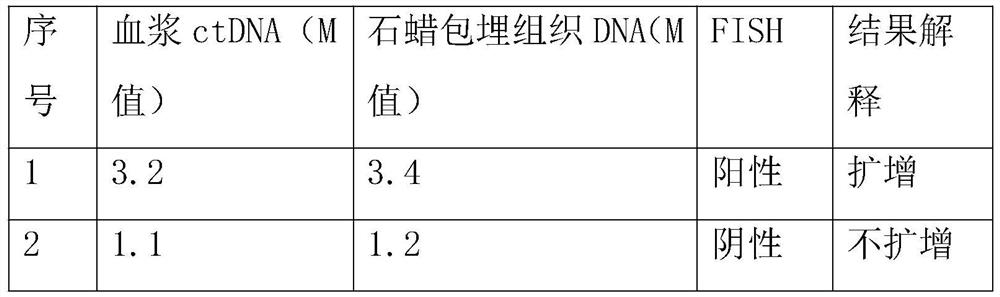
[0057] 1. Based on ddPCR technology, compare the consistency of ERBB2 gene amplification between plasma ctDNA and tumor tissue DNA in patients with gastric cancer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com