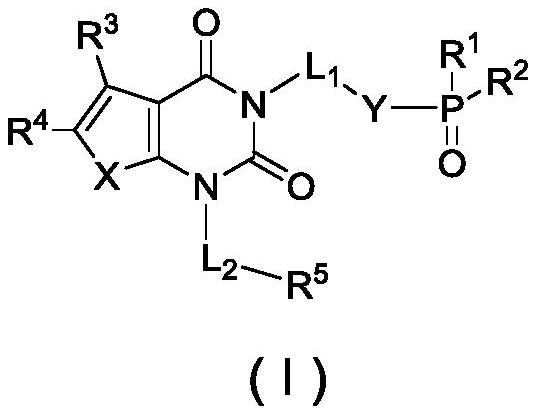
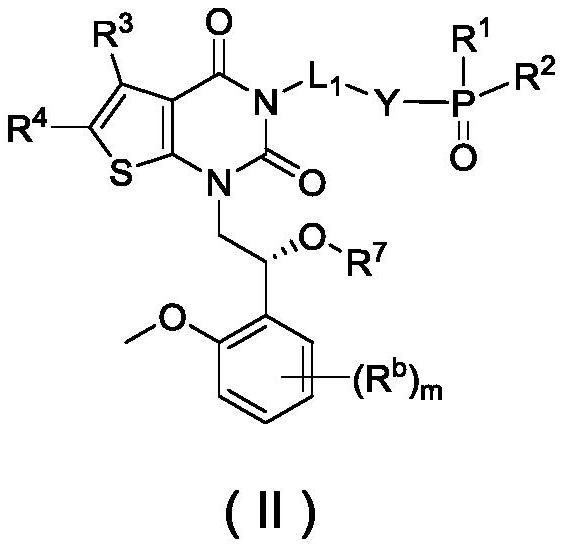
Phosphoric acid or phosphate derivatives, preparation method and medical application thereof
A compound, selected technology, applied in the field of metabolic related disorders and diseases, which can solve problems such as unsatisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
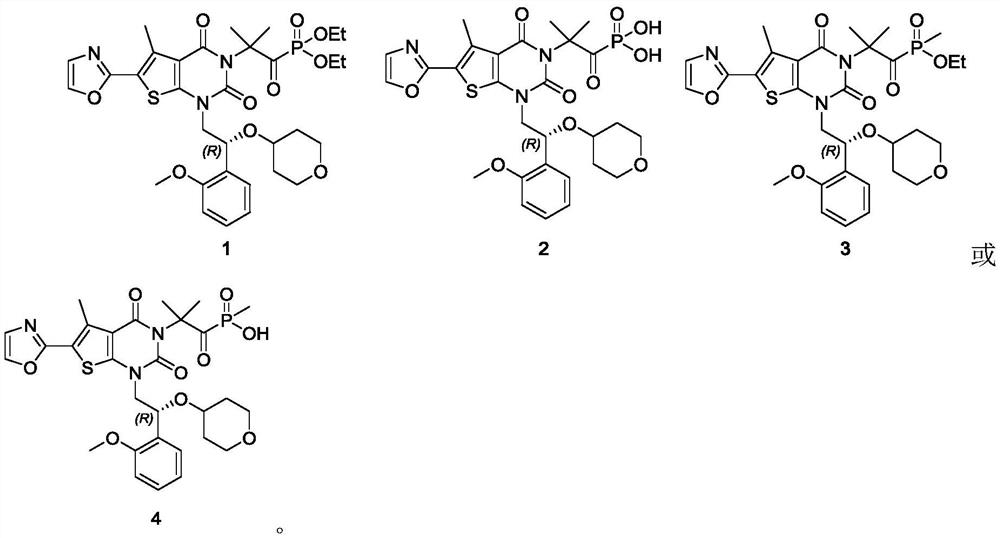
[0083] (R)-[2-[1-[2-(2-methoxyphenyl)-2-[(tetrahydro-2H-pyran-4--yl)oxy]ethyl]-5-methyl Base-6-(oxazol-2-yl)-2,4-dioxo-1,4-dihydrothieno[2,3-d]pyrimidin-3(2H)-yl]-2-methyl Propionyl] diethyl phosphate (compound 1)
[0084] Diethyl
[0085] (R)-(2-(1-(2-(2-methoxyphenyl)-2-((tetrahydro-2H-pyran-4-yl)oxy)ethyl)-5-methyl-6-(oxazol-2-yl )-2,4-dioxo-1,4-dihydrothieno[2,3-d]pyrimidin-3(2H)-yl)-2-methylpropanoyl)phosphonate(1)
[0086]
[0087] first step:
[0088] (R)-2-[1-[2-(2-methoxyphenyl)-2-[(tetrahydro-2H-pyran-4-yl)oxy]ethyl]-5-methyl- 6-(oxazol-2-yl)-2,4-dioxo-1,4-dihydrothieno[2,3-d]pyrimidin-3(2H)-yl]-2-methylpropionyl chloride (Compound 1B)
[0089] (R)-2-(1-(2-(2-methoxyphenyl)-2-((tetrahydro-2H-pyran-4-yl)oxy)ethyl)-5-methyl-6-(oxazol-2-yl) -2,4-dioxo-1,4-dihydrothieno[2,3-d]pyrimidin-3(2H)-yl)-2-methylpropanoyl chloride(1B)
[0090] Compound (R)-2-[1-[2-(2-methoxyphenyl)-2-[(tetrahydro-2H-pyran-4-yl)oxy]ethyl]-5-methyl Base-6-(oxazol-2-yl)-2,4-dioxo-1,4-dih...
Embodiment 2
[0100] (R)-[2-[1-[2-(2-methoxyphenyl)-2-[(tetrahydro-2H-pyran-4--yl)oxy]ethyl]-5-methyl Base-6-(oxazol-2-yl)-2,4-dioxo-1,4-dihydrothieno[2,3-d]pyrimidin-3(2H)-yl]-2-methyl propionyl]phosphate (compound 2)
[0101] (R)-(2-(1-(2-(2-methoxyphenyl)-2-((tetrahydro-2H-pyran-4-yl)oxy)ethyl)-5-methyl-6-(oxazol-2-yl )-2,4-dioxo-1,4-dihydrothieno[2,3-d]pyrimidin-3(2H)-yl)-2-methylpropanoyl)phosphonic acid(2)
[0102]
[0103] Compound 1 (70 mg, 0.1 mmol) was dissolved in 2 mL of acetonitrile, trimethylbromosilane (47 mg, 0.3 mmol) was added dropwise, and reacted at 65° C. for 2 hours. After the reaction was completed, it was concentrated under reduced pressure to obtain compound 2 as a light yellow solid (60 mg, 0.1 mmol, yield 99%).
[0104] 1 H NMR (400MHz, d6-DMSO): δ8.26(s, 1H), 7.50(dd, J=7.6, 1.6Hz, 1H), 7.41(s, 1H), 7.31(t, J=7.2Hz, 1H ),6.95–7.05(m,2H),5.26-5.30(m,1H),4.11–4.23(m,2H),3.86(s,3H),3.35-3.75(m,5H),3.20-3.30(m ,2H),2.85(s,3H),1.68-1.83(m,8H),1.31-1.52(m,2H)....
Embodiment 3
[0108] [2-[1-[(R)-2-(2-methoxyphenyl)-2-[(tetrahydro-2H-pyran-4-yl)oxy]ethyl]-5-methyl -6-(oxazol-2-yl)-2,4-dioxo-1,4-dihydrothieno[2,3-d]pyrimidin-3(2H)-yl]-2-methylpropane Acyl] ethyl methyl phosphate (compound 3)
[0109] Ethyl
[0110] (2-(1-((R)-2-(2-methoxyphenyl)-2-((tetrahydro-2H-pyran-4-yl)oxy)ethyl)-5-methyl-6-(oxazol-2-yl )-2,4-dioxo-1,4-dihydrothieno[2,3-d]pyrimidin-3(2H)-yl)-2-methylpropanoyl)(methyl)phosphinate(3)
[0111]
[0112] Compound 1B (2.06g, 3.5mmol) was dissolved in 20ml of dichloromethane, diethyl methylphosphonite (0.96g, 7.0mmol) was slowly added dropwise at 10°C, reacted overnight at room temperature, concentrated under reduced pressure, and the residue The product was separated and purified by silica gel column chromatography (eluent: system B). Compound 3 was obtained as a white solid (1.3 g, 2.0 mmol, yield 56%).
[0113] 1 H NMR (400MHz, CDCl 3 ):δ7.71(s,1H),7.59(dd,J=7.6,1.2Hz,1H),7.26-7.30(m,1H),7.22(s,1H),7.03–7.04(m,1H), 6.85-6.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com