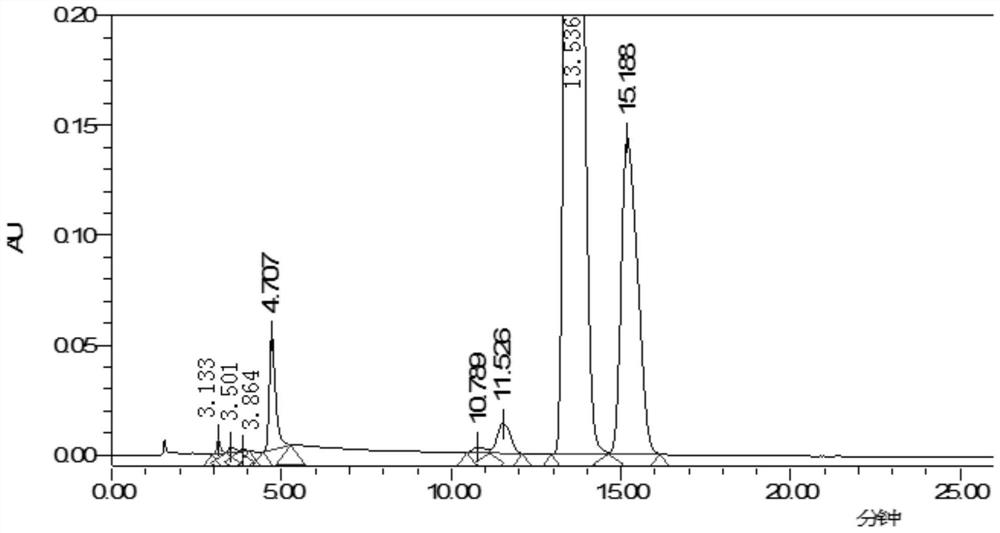
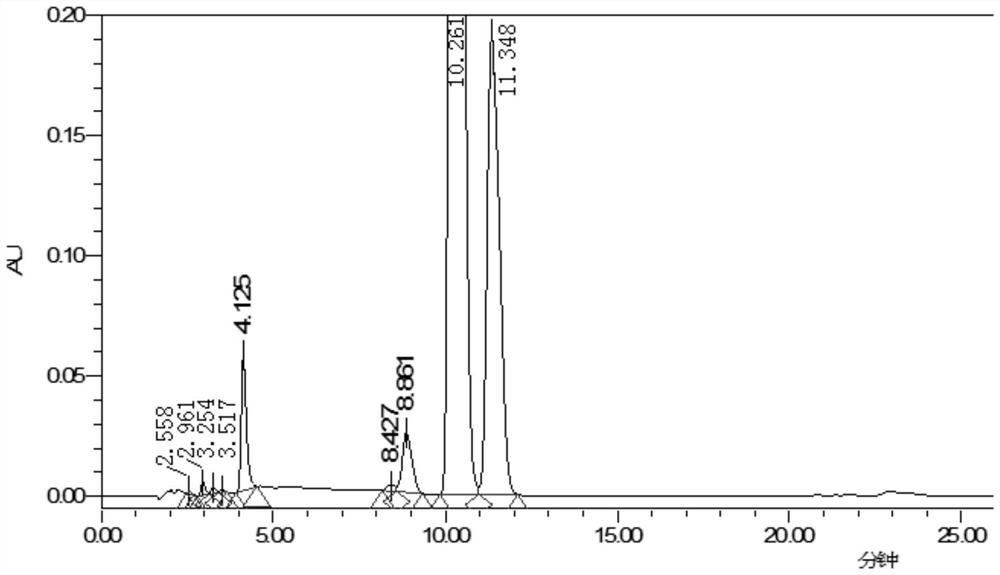
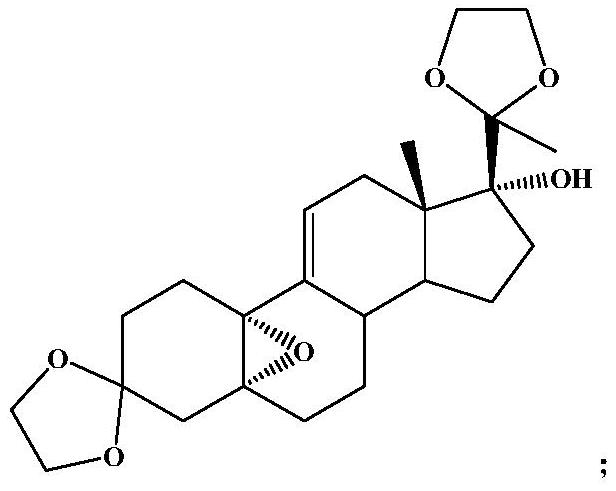
Method for detecting content of 3, 3, 20, 20-bis(ethylenedioxy)-17alpha-hydroxy-19-norpregna-5(10),9(11)-diene in ulipristal acetate intermediate I
A technology of ulipristal acetate and intermediates, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as difficulties, and achieve the effect of simple operation, good selectivity, and better separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A method for detecting ulinastal ketal content in ulipristal acetate intermediate I, comprising the steps,
[0026] 1. The chromatographic column is Welch Ultimate XB-C18 250*4.6mm, 5μm particle size or similar chromatographic column.
[0027] 2. The detection wavelength is 235nm, which is the wavelength at which ulipristal acetate intermediate Ⅰ and ulinacetal have relatively large absorption in the ultraviolet-visible region.
[0028] 3. Through the optimized gradient elution procedure, as follows:
[0029]
[0030] 4. The analysis time is 2.0 times the retention time of the main peak.
[0031] 5. Use the area normalization method to calculate the content of ulipristal acetate intermediate I; the injection volume is 10ul; the flow rate is 1.0ml / min; the column temperature is 35°C.
[0032] 6. Orthodione positioning solution: Accurately weigh an appropriate amount of ureadione, accurately weigh it, dissolve and dilute it with acetonitrile to make a solution contai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com