Pharmaceutical composition for treating deficiency fire and phlegm stagnation syndrome lymphatic system diseases
A composition and a technology of traditional Chinese medicine, applied in the field of traditional Chinese medicine compositions, can solve the traditional Chinese medicine composition that does not see the disease of the lymphatic system with deficiency of fire and phlegm, does not record the specific syndrome type of the lymphatic system disease, and does not record the compatibility relationship between the ruler, minister, assistant and envoy of the traditional Chinese medicine composition, etc. problem, to achieve excellent therapeutic effect, less toxic and side effects, and high cure rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1 pharmaceutical composition of the present invention
[0044] 1. Prescription
[0045] Composition a: 300g Tiankuizi, 300g Prunella vulgaris, 450g white ground cucumber, 250g Polygonum multiflorum, 200g honeysuckle;
[0046] Composition b: 400g of icegrass, 300g of Prunella vulgaris, 100g of seaweed, 200g of Nocturna vine, 150g of Schizonepeta, 200g of Lonicera vine, 100g of Akebia.
[0047] 2. Preparation method
[0048] (1) According to the prescription amount of composition a, take tiankuizi, Prunella vulgaris, cucumber, Radix Polygoni Multiflori, and honeysuckle, and pass through a 100-mesh sieve after crushing to obtain powder;
[0049] (2) According to the prescription quantity of composition b, take Lingbingcao, Prunella vulgaris, seaweed, night cross vine, nepeta, honeysuckle vine, akebia, add 2-3 times the weight of water, bring to a boil with high heat, and simmer for 1- After 2 hours, filter and collect the decoction; add 2 / 3...
experiment example 1
[0052] Clinical case statistics of experimental example 1 pharmaceutical composition of the present invention to lymphadenitis therapeutic effect
[0053] 1. TCM diagnostic criteria
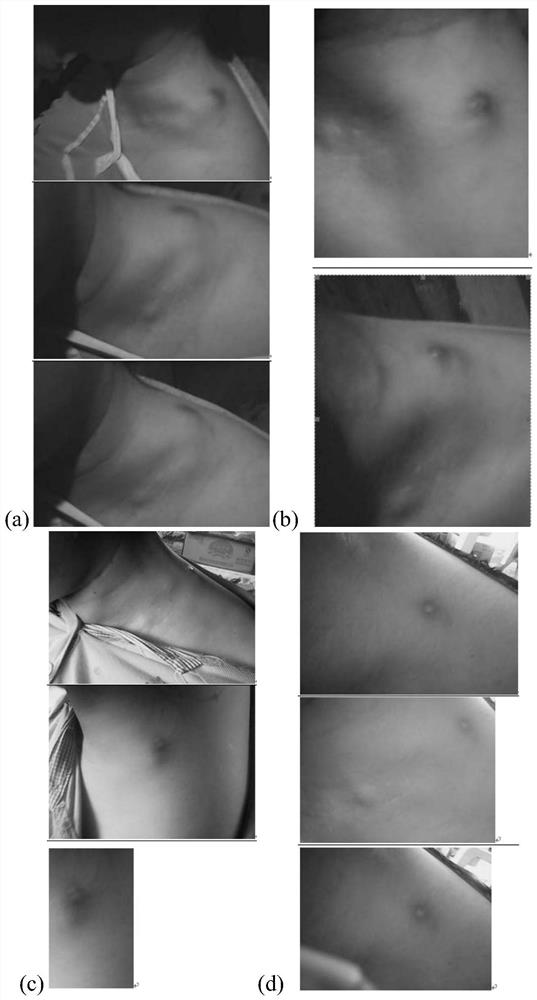
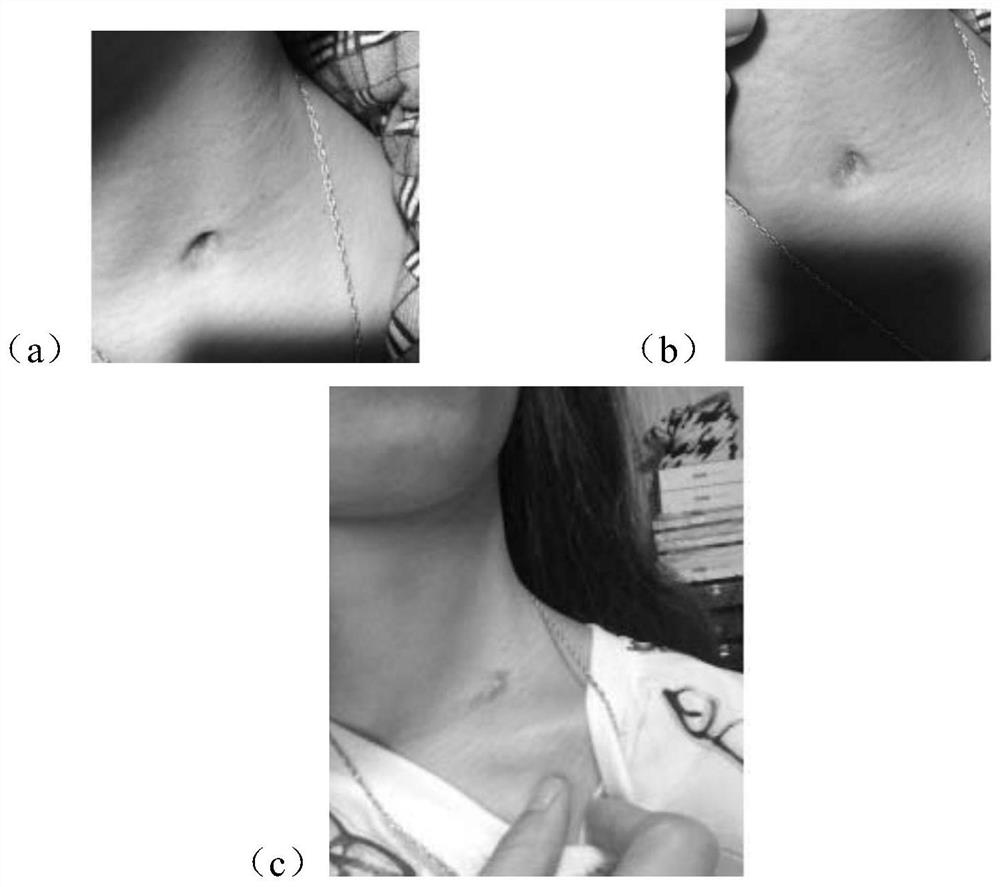
[0054] Deficiency-fire phlegm syndrome lymphitis: multiple swollen nuclei in the neck, under the ear or under the armpits, no pain or itching, unchanged skin color, dizziness and tinnitus, or accompanied by bitter mouth and dry throat, or yellow and white phlegm, chest and abdomen distension, Dry stool, short red urine, red tongue with yellow fur, stringy pulse. The swollen nuclei are as large as soybeans, one or several, which can appear simultaneously or successively. The skin color does not change, the texture is slightly hard, the surface is smooth, neither hot nor painful, and can be moved when pushed.
[0055] 2. Standard of treatment
[0056] Cure standard: Symptoms disappear completely.
[0057] Effective standard: more than 80% of symptoms disappear.
[0058] 3. Treatment method
[...
experiment example 2
[0062] Experimental example 2 the clinical case statistics of pharmaceutical composition of the present invention to lymphatic tuberculosis therapeutic effect
[0063] 1. TCM diagnostic criteria
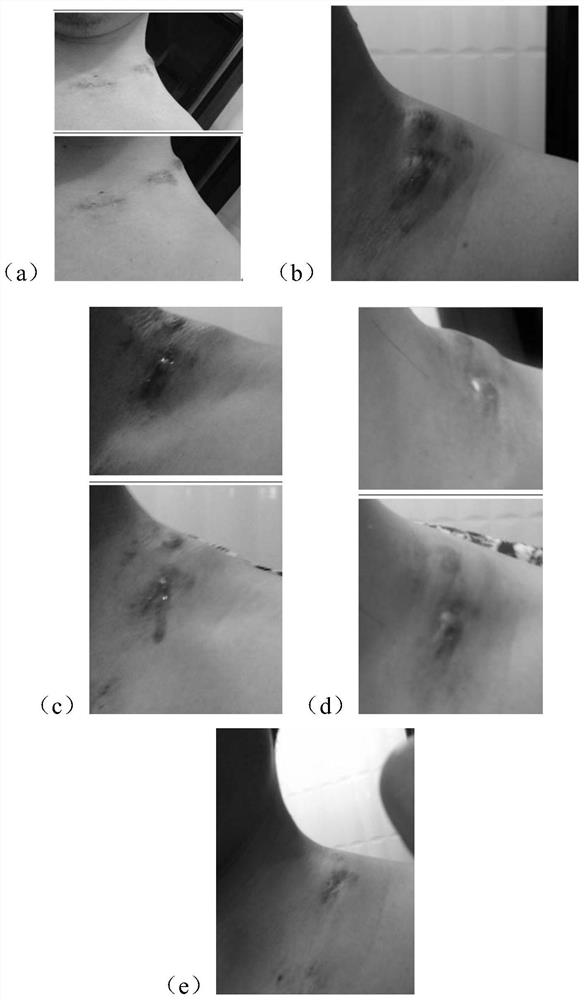
[0064] Deficiency-fire phlegm syndrome lymphatic tuberculosis: multiple swollen nuclei in the neck, under the ear or under the armpit, painless or itchy, skin color unchanged, dizziness and tinnitus, or accompanied by bitter mouth and dry throat, or yellow and white phlegm, chest and abdomen distension, Dry stool, short red urine, red tongue with yellow fur, stringy pulse. The nuclear mass adheres to the epidermis, and sometimes several nuclear masses fuse with each other to form a large mass, which cannot be moved and is painful when pushed. When further purulent, the superficial skin turns dark red, slightly hot, and there is a slight fluctuating sensation when pressed.
[0065] 2. Standard of treatment
[0066] Cure standard: Symptoms disappear completely.
[0067] Effective s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com