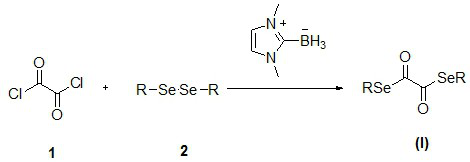
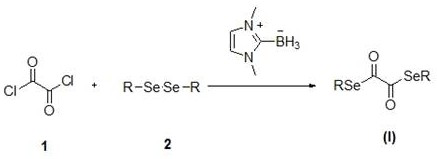
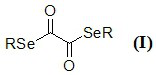
Diselenide oxalate compound and its synthesis method and application
A technology of ester compound and synthesis method, which is applied in the direction of organic chemistry, organic chemistry, etc., can solve the problem of limited selenium alkylation reagents, and achieve the effects of high atom utilization, simple operation, and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In a round bottom flask add 1.2 mmol dimethyldiselenide, 1.2 mmol N-methylimidazolium salt, 3 mL CH 3 CN, placed in a 100°C oil bath and stirred for 12 h to stop the reaction. The reaction solution was cooled to room temperature, and 1 mmol oxalyl chloride was added quickly, and the reaction was stopped after 2 h of reaction. The solvent was removed by rotary evaporation under reduced pressure, and the residue was purified by flash silica gel column to obtain methyl diselenide oxalate with a yield of 75%.
[0027]
[0028] Compound 3a: yellow solid; melting point: 85-86 o C; Eluant: ethyl acetate / petroleumether (1:100, R f = 0.30). 1 H NMR (400 MHz, CDCl 3 ) δ 2.33 – 2.30 (m, 6H); 13 CNMR (101 MHz, CDCl 3 ) δ 193.9 (2C), 4.7 (2C). IR (KBr): ν = 2954, 2920, 2850, 1671, 1461, 1377, 927 cm -1 . HRMS (ESI) for C 4 h 6 o 2 Se 2 Na (M+Na) + : Calcd: 268.8590, Found: 268.8579.
[0029] Using N,N-dimethylformamide instead of acetonitrile, and other conditions u...
Embodiment 2
[0031] Add 1.2 mmol diethyldiselenide, 1.2 mmol N-methylimidazolium salt, 3 mL CH 3 CN, placed in a 90°C oil bath and stirred for 12 h to stop the reaction. The reaction solution was cooled to room temperature, and 1 mmol oxalyl chloride was added quickly, and the reaction was stopped after 2 h of reaction. The solvent was removed by rotary evaporation under reduced pressure, and the residue was purified by flash silica gel column to obtain ethyl diselenide oxalate with a yield of 78%.
[0032]
[0033] Compound 3b: yellow liquid; Eluant: ethyl acetate / petroleum ether (1:100, R f = 0.30). 1 H NMR (400 MHz, CDCl 3 ) δ 3.00 (q, J = 7.5 Hz, 4H), 1.46 (t, J = 7.5Hz, 6H); 13 C NMR (101 MHz, CDCl 3 ) δ 194.3 (2C), 19.2 (2C), 15.3 (2C). IR(KBr): ν = 2955, 2919, 2849, 1693, 1647, 1468, 1377, 1275, 1261, 1018, 764,750 cm -1 . HRMS (ESI) for C 6 h 10 o 2 Se 2 Na (M+Na) + : Calcd: 296.8903, Found: 296.8916.
[0034] Using 1,2-dichloroethane instead of acetonitrile, and...
Embodiment 3
[0036] In a round bottom flask add 1.2 mmol dipentyl diselenide, 1.2 mmol N-methylimidazolium salt, 3 mL CH 3 CN, placed in an 80°C oil bath and stirred for 15 h to stop the reaction. The reaction solution was cooled to room temperature, and 1 mmol oxalyl chloride was added quickly, and the reaction was stopped after 3 h of reaction. The solvent was removed by rotary evaporation under reduced pressure, and the residue was purified by flash silica gel column to obtain diselenyl oxalate with a yield of 74%.
[0037]
[0038] Compound 3c: yellow liquid; Eluant: ethyl acetate / petroleum ether (1:100, R f = 0.30). 1 H NMR (400 MHz, CDCl 3 ) δ 3.00 (t, J = 7.4 Hz, 4H), 1.75 – 1.67 (m,4H), 1.41 – 1.31 (m, 8H), 0.90 (t, J = 6.9 Hz, 6H); 13 C NMR (101 MHz, CDCl 3) δ194.1 (2C), 32.1 (2C), 29.6 (2C), 25.3 (2C), 22.2 (2C), 14.0 (2C). IR (KBr):ν = 2956, 2920, 2850, 1691, 1647, 1468, 1378, 1275, 764, 750, 721, 700 cm -1 .HRMS (ESI) for C 12 h 22 o 2 Se 2 Na (M+Na) + : Calc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com