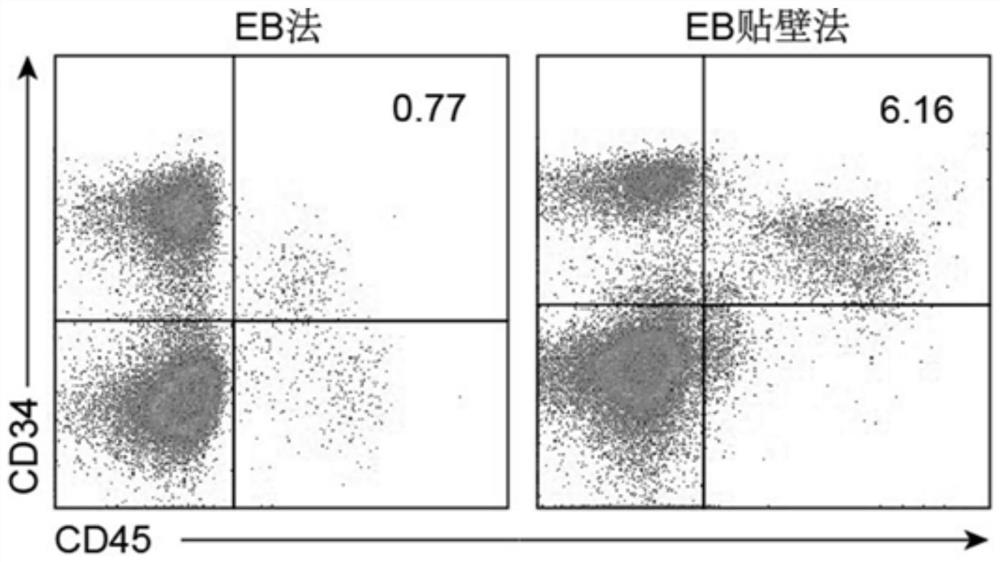
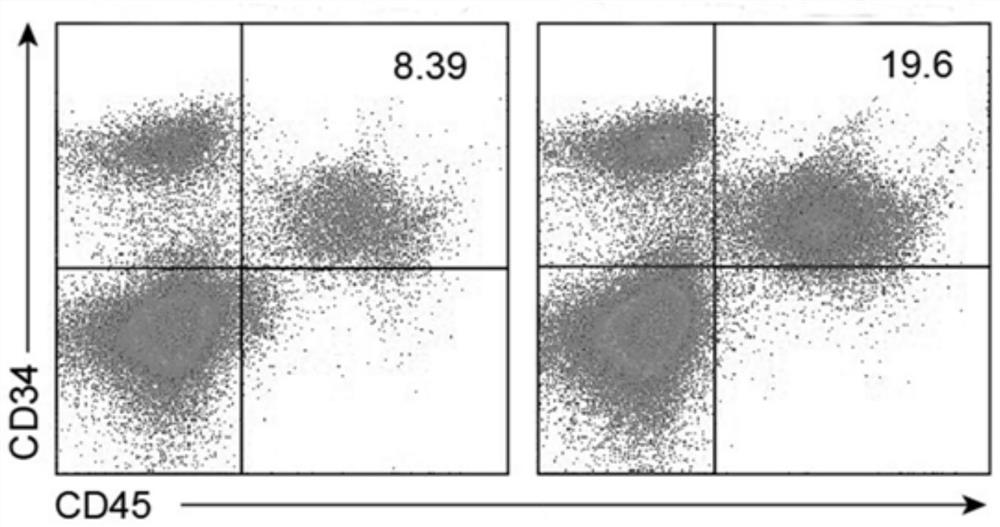
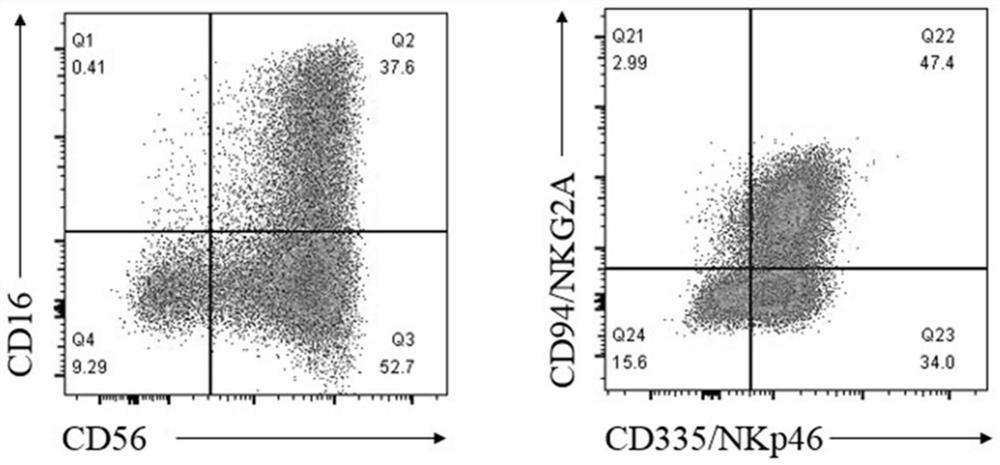
Natural killer cell differentiated from human pluripotent stem cells as well as preparation method and application thereof
A natural killer cell and human pluripotent stem cell technology, applied in the field of stem cell biology, can solve problems such as complex operation and low efficiency of NK cell system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] A method for directionally inducing differentiation of human pluripotent stem cells into natural killer cells, comprising the following steps:
[0085] S1, forming embryoid bodies;
[0086] S2. Directed permanent hematopoietic differentiation of embryoid bodies to obtain hematopoietic precursor cells;
[0087] S3. Hematopoietic precursor cells differentiate to obtain natural killer cells;
[0088] S4: Expansion of natural killer cells.
[0089] Specific steps are as follows:
[0090] 1. Day-1 to day0: formation of embryoid bodies
[0091] The well-growing hPSCs were digested into single cells, resuspended in human pluripotent stem cell culture medium, and allowed to stand overnight in the presence of anti-apoptosis inhibitors to form EB spheres with uniform size and shape. The hPSCs used in the experiment are hiPSCs and commercialized hESCs, which have undergone strict pluripotency verification and are maintained in normal human pluripotent stem cell medium E8.
[...
Embodiment 2
[0125] A method for directionally inducing differentiation of human pluripotent stem cells into natural killer cells, the specific steps are as follows:
[0126] 1. Day-1 to day0: formation of embryoid bodies
[0127] The well-growing hPSCs were digested into single cells, resuspended in human pluripotent stem cell culture medium, and allowed to stand overnight in the presence of anti-apoptosis inhibitors to form EB spheres with uniform size and shape. The hPSCs used in the experiment are hiPSCs and commercialized hESCs, which have undergone strict pluripotency verification and are maintained in the normal human pluripotent stem cell medium mTeSR.
[0128] The hPSCs cultured by the above method were subjected to the embryoid body formation experiment when the growth density was about 80%. The specific method is: use Accutase to completely digest hPSCs into a single cell suspension, and add the anti-apoptosis inhibitor HA100, the concentration of the inhibitor is 1 μM, and the...
Embodiment 3
[0153] A method for directionally inducing differentiation of human pluripotent stem cells into natural killer cells, the specific steps are as follows:
[0154] 1. Day-1 to day0: formation of embryoid bodies
[0155] The well-growing hPSCs were digested into single cells, resuspended in human pluripotent stem cell culture medium, and allowed to stand overnight in the presence of anti-apoptosis inhibitors to form EB spheres with uniform size and shape. The hPSCs used in the experiment are hiPSCs and commercialized hESCs, which have undergone strict pluripotency verification and are maintained in the normal human pluripotent stem cell medium mTeSR.
[0156] The hPSCs cultured by the above method were subjected to the embryoid body formation experiment when the growth density was about 80%. The specific method is: use EDTA to completely digest hPSCs into a single cell suspension, and add the anti-apoptosis inhibitor Blebbistatin, the concentration of the inhibitor is 50 μM, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com