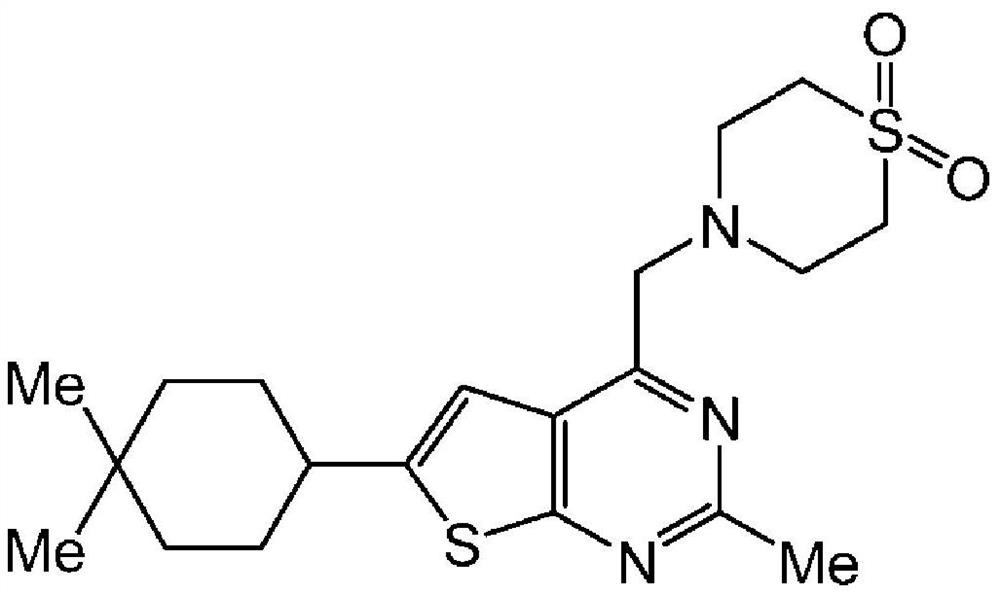
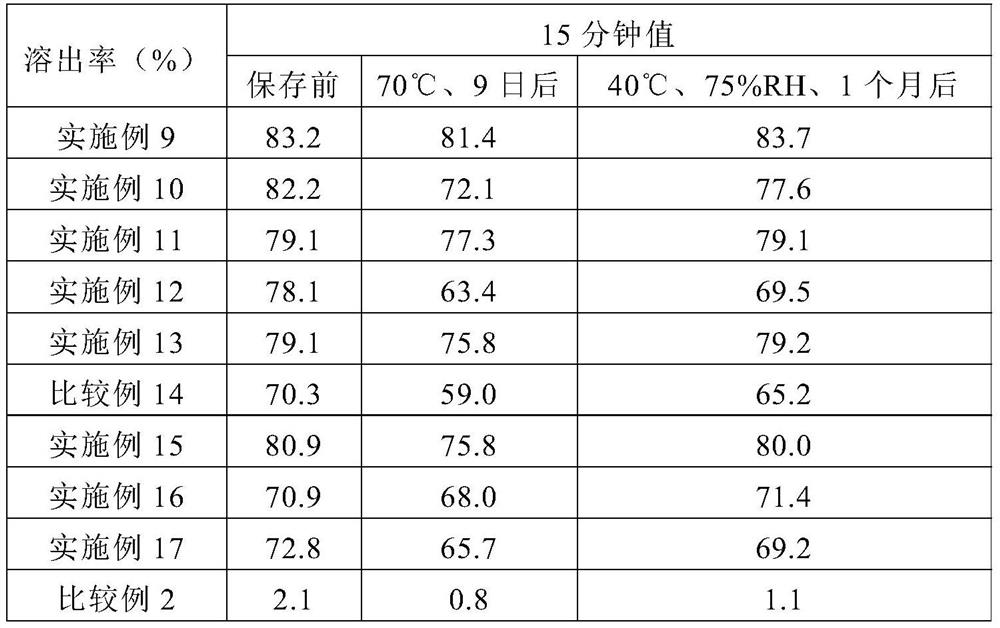
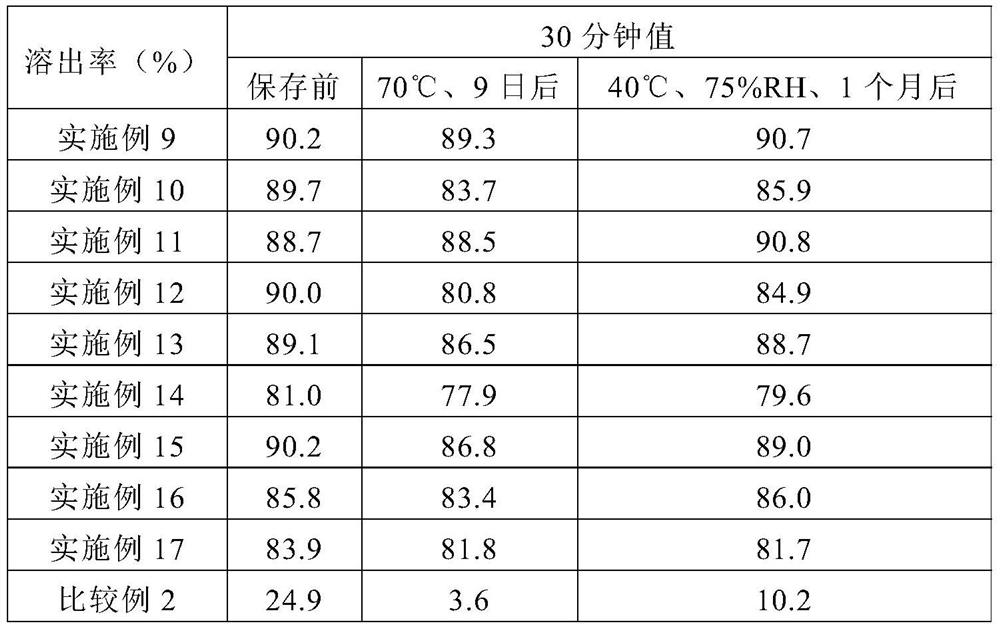
Pharmaceutical composition for oral administration
A pharmaceutical composition and oral technology, which are applied in the field of pharmaceutical compositions for oral administration, can solve the problems of effectiveness or quick-acting effect, reduction of drug amount, etc., and achieve the effect of rapid drug dissolution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] 1 part by weight of Compound A and 99 parts by weight of L-HPC99 were put into a glass bottle, capped, and shaken vigorously by hand to make a physical mixture.
Embodiment 2
[0126] According to the formulation in Table 2, 1.00 parts by weight of pulverized compound A, 69.20 parts by weight of lactose hydrate, 27.00 parts by weight of crystalline cellulose (UF-711), 27.00 parts by weight of L-HPC, 2.70 parts by weight of Light anhydrous silicic acid in parts by weight, sodium stearyl fumarate in 6.75 parts by weight, and magnesium stearate in 1.35 parts by weight were mixed to obtain a mixture before tableting. The obtained pre-tabletting mixture was shaped using a tableting machine to obtain round tablets weighing 135.00 mg and having a diameter of 7 mm.
Embodiment 3
[0128] According to the formula in Table 2, 25.00 parts by weight of pulverized compound A, 326.00 parts by weight of lactose hydrate, 135.00 parts by weight of crystalline cellulose (UF-711), 135.00 parts by weight of L-HPC, 13.50 parts by weight of Light anhydrous silicic acid in parts by weight, sodium stearyl fumarate in 33.75 parts by weight, and magnesium stearate in 6.75 parts by weight were mixed to obtain a mixture before tableting. The obtained pre-tabletting mixture was molded using a tableting machine to obtain oval tablets with a weight of 675.00 mg and a major diameter of 16 mm×short diameter of 8 mm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com