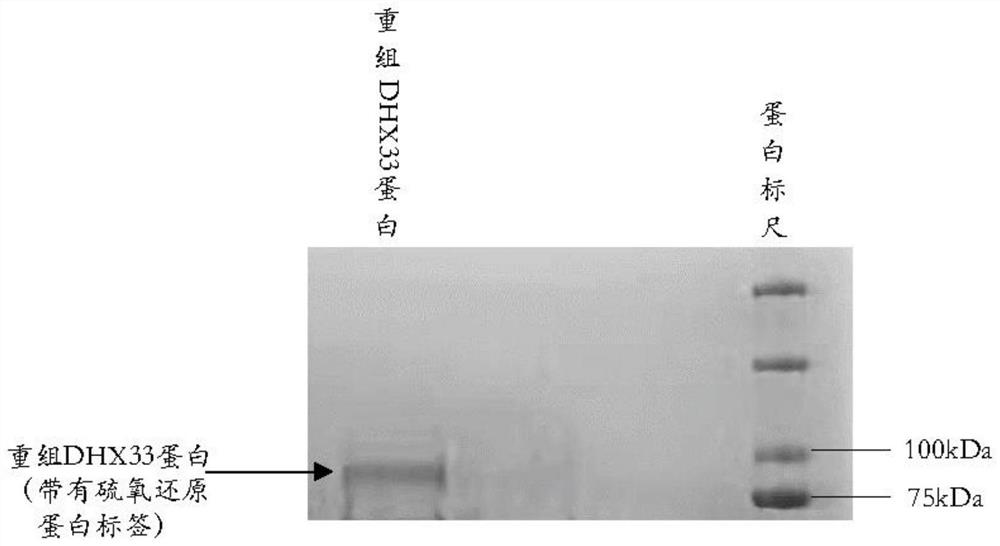
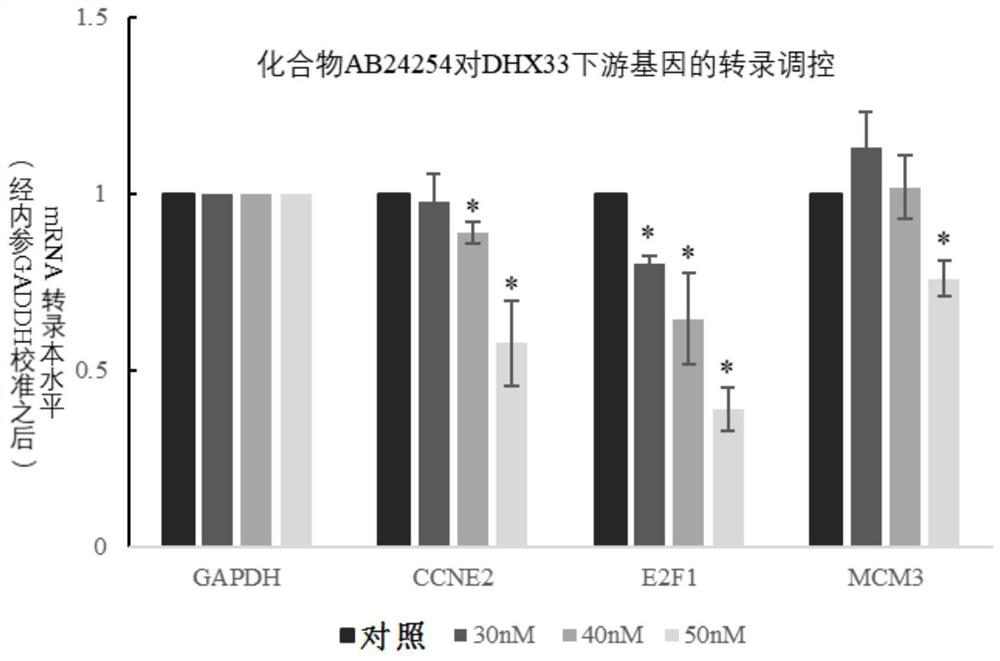
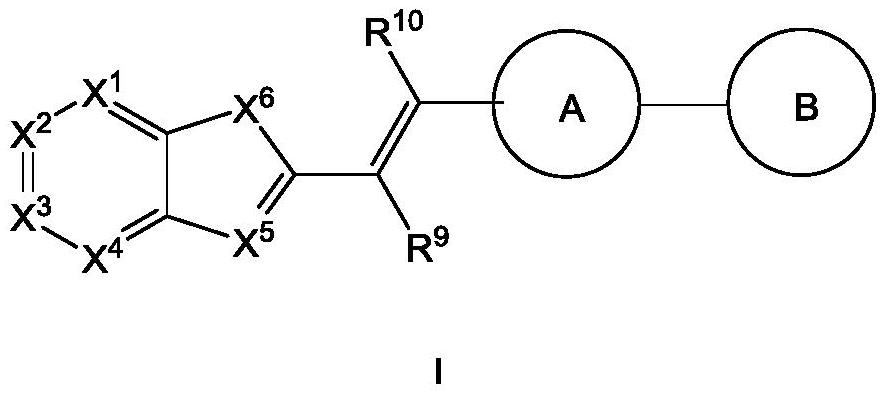
Polycyclic compound for inhibiting RNA helicase DHX33
A compound, CH3 technology, applied in organic chemistry, drug combination, medical preparations containing active ingredients, etc., can solve the problem that mutants lacking helicase activity do not have the function of DHX33 protein, cannot replace the function, and are rare in small molecule inhibitors And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Example 1: Compound (E)-2-(3-(2-cyano-2-(3H-imidazo[4,5-c]pyridin-2-yl)vinyl)-2,5-dimethyl Synthesis of 1H-pyrrol-1-yl)-4,5-dimethylfuran-3-carbonitrile (AB24249)
[0140] (1) Synthesis of compound 3 (2-(3H-imidazo[4,5-c]pyridin-2-yl)acetonitrile)
[0141]
[0142] Compound 1 (3,4-diaminopyridine) (400 mg, 3.67 mmol, 1.0 eq) and compound 2 (ethyl cyanoacetate) (1.24 g, 11.01 mmol, 3.0 eq) were dissolved in dimethylformamide (6 mL ), stirred at 180°C for 5 hours. After the reaction was cooled, the solvent was removed. The residue was purified by flash column chromatography (dichloromethane:methanol=50:1 to 10:1) to obtain brown solid powder compound 3 (2-(3H-imidazo[4,5-c]pyridin-2-yl) Acetonitrile) (180 mg, yield: 31.0%). MS(ESI)m / z:159[M+H] + . TLC: DCM / MeOH (10:1); R f (Compound 1) = 0.1; R f (compound 3) = 0.3.
[0143] (2) Compound AB24249 ((E)-2-(3-(2-cyano-2-(3H-imidazo[4,5-c]pyridin-2-yl)vinyl)-2,5-di Synthesis of methyl-1H-pyrrol-1-yl)-4,5-dimethylf...
Embodiment 2
[0146] Example 2: Compound (E)-2-(3-(2-cyano-2-(6-methoxy-3H-imidazo[4,5-c]pyridin-2-yl)vinyl)- Synthesis of 2,5-Dimethyl-1H-pyrrol-1-yl)-4,5-Dimethylfuran-3-carbonitrile (AB24254)
[0147] (1) Synthesis of compound 6 (6-methoxypyridine-3,4-diamine)
[0148]
[0149] Compound 5 (2-methoxy-5-nitropyridin-4-amine) (3.0 g, 17.74 mmol, 1.0 eq) was dissolved in methanol (30 mL), and a carbon-supported palladium catalyst (300 mg, 0.1 wt %) was added . The mixture was stirred at room temperature under hydrogen for 16 hours. The solid was filtered, and then the filtrate was concentrated to obtain a brown solid compound 6 (6-methoxypyridine-3,4-diamine) (2.6 g, yield: 100%). MS(ESI)m / z:140[M+H] + . TLC: DCM:MeOH (10:1); Rf (compound 5) = 0.7; Rf (compound 6) = 0.5.
[0150] (2) Synthesis of compound 7 (2-(6-methoxy-3H-imidazo[4,5-c]pyridin-2-yl)acetonitrile)
[0151]
[0152] Compound 6 (6-methoxypyridine-3,4-diamine) (1.5g, 10.2mmol, 1.0eq) and compound 2 (ethyl cyanoacet...
Embodiment 3
[0156] Example 3: Compound (E)-2-(3-(2-cyano-2-(6-methoxy-3H-imidazo[4,5-c]pyridin-2-yl)vinyl)- Synthesis of 2,5-Dimethyl-1H-pyrrol-1-yl)-5-methylthiophene-3-carbonitrile (AB24288)
[0157] AB24288 was synthesized in the same manner as in Example 2 (yield: 13.6%), 1 H NMR(400MHz,DMSO-d6)δ8.53(s,1H),8.16(s,1H),7.31(s,1H),6.96(s,1H),6.89(s,1H),3.89(s, 3H), 2.51(s,3H), 2.30(s,3H), 2.08(s,3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com