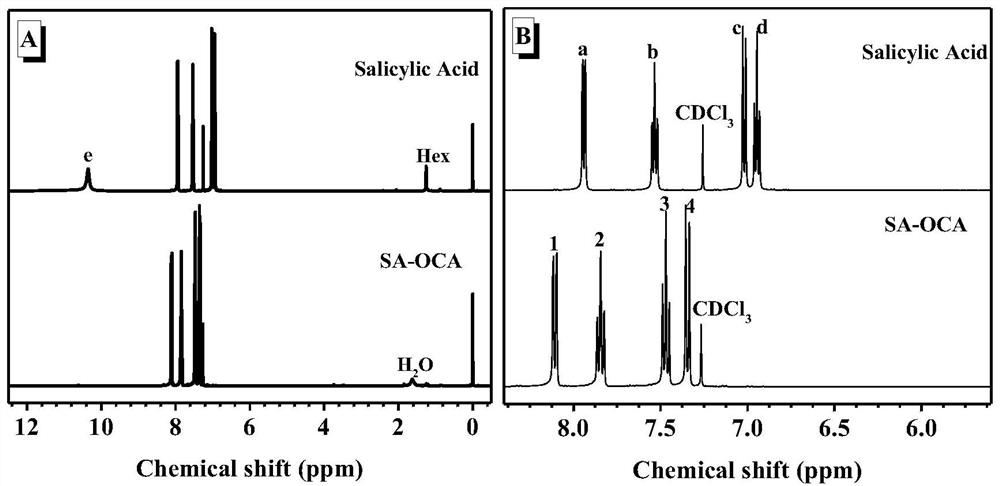
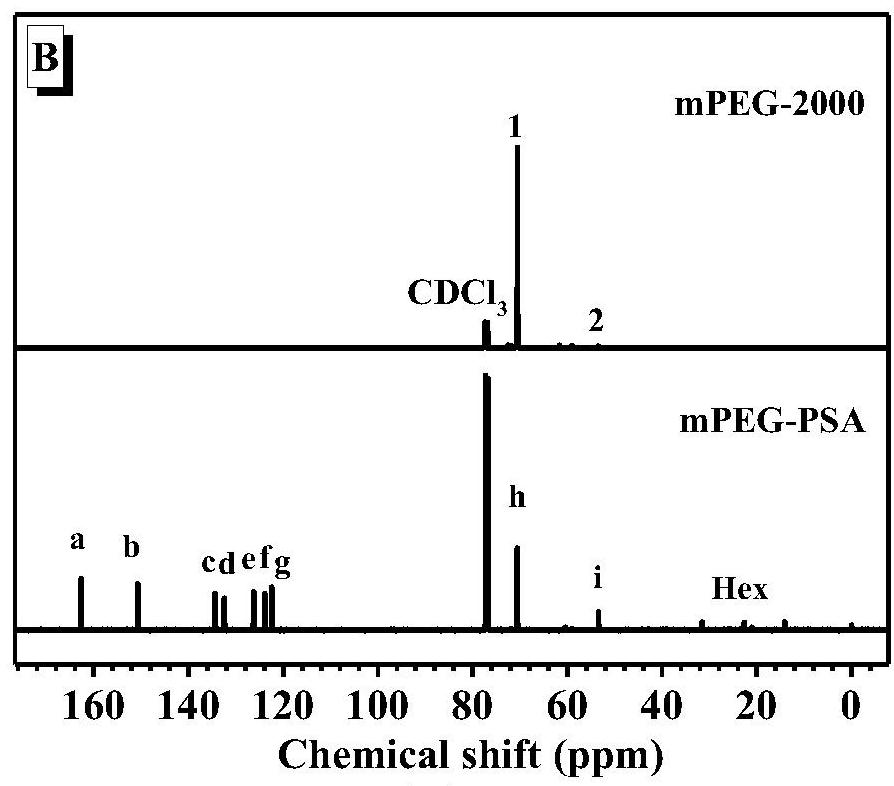
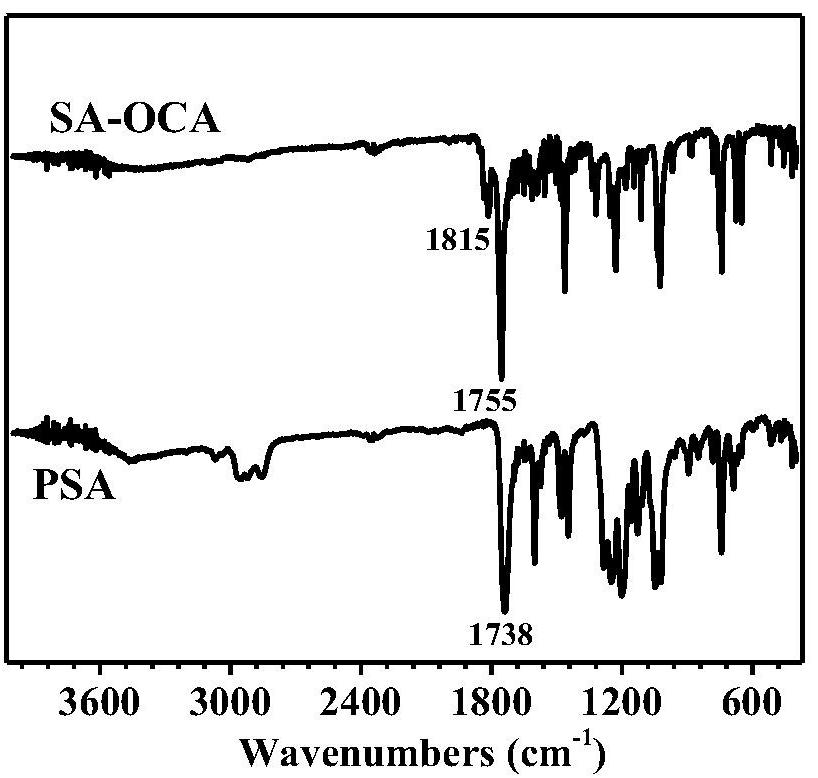
Method for synthesizing polysalicylate through ring-opening polymerization
A salicylate, ring-opening polymerization technology, applied in the field of polyester synthesis, can solve the problems of poor controllability, low reaction efficiency, high production cost of melt polycondensation, and achieve the effect of narrow molecular weight distribution and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add the salicylic acid solution dropwise to the triphosgene solution at low temperature, after a period of reaction, add the triethylamine solution dropwise, and take it out to normal temperature. After the reaction was completed, it was filtered with suction and concentrated to obtain a crude product. The crude product was recrystallized to obtain salicylic acid cyclic anhydride (SA-OCA) monomer in white crystals.
Embodiment 2
[0028] Add 50 equivalents of SA-OCA monomer and 1 ml of tetrahydrofuran to the reaction tube, stir for 10 minutes, add 1 equivalent of DBU, 1 equivalent of benzyl alcohol, and react at room temperature. After 5 minutes, the conversion rate reaches 95% or more, then stop After reaction, a white polymer was obtained by precipitation. Gel permeation chromatography (GPC) result shows that gained polymer molecular weight M n =4.52KDa, molecular weight distribution
Embodiment 3
[0030] Add 50 equivalents of SA-OCA monomer and 1 ml of tetrahydrofuran to the reaction tube, stir for 10 minutes, add 1 equivalent of DBN, 1 equivalent of benzyl alcohol, and react at room temperature. After 5 minutes, the conversion rate reaches more than 95% and then stop After reaction, a white polymer was obtained by precipitation. Gel permeation chromatography (GPC) result shows that gained polymer molecular weight M n =2.54KDa, molecular weight distribution
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com