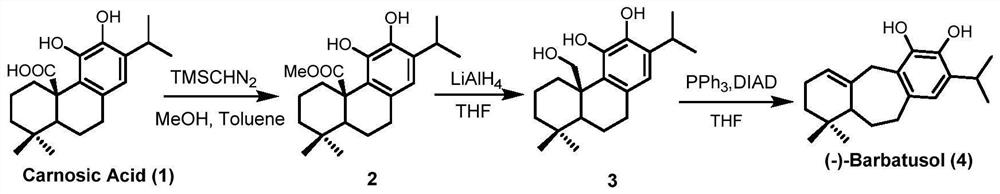
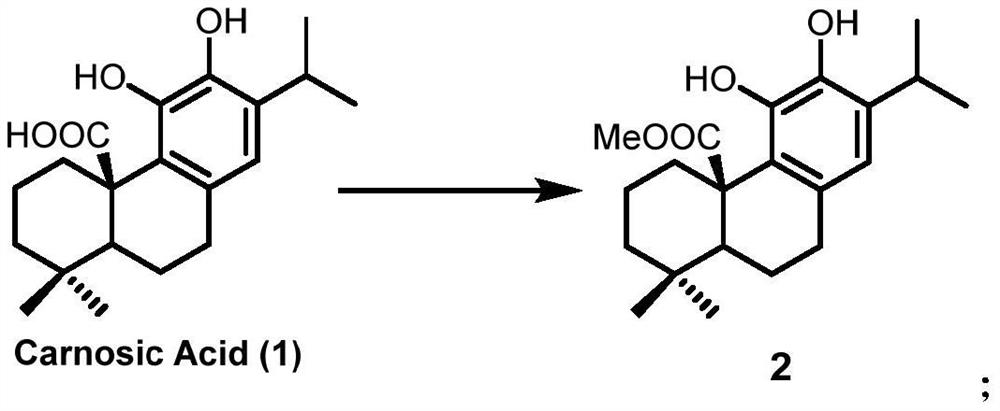
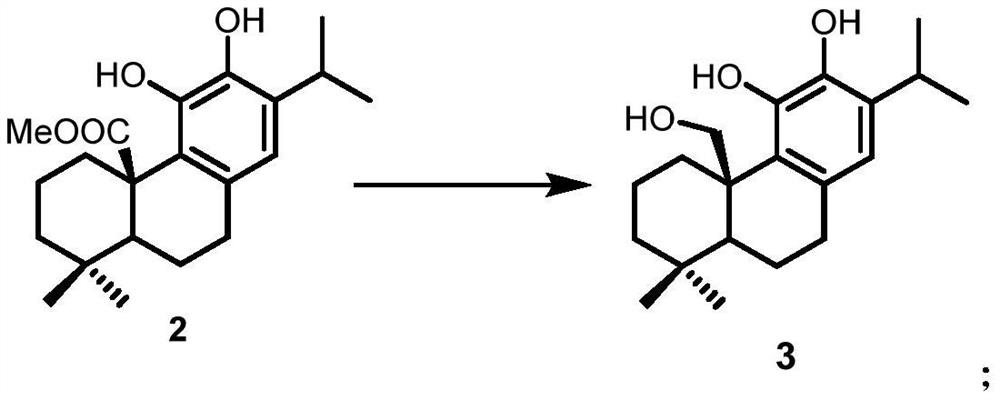
A kind of preparation method of icetexane type abietane diterpene
A technology of rosinane diterpene and diazomethane, which is applied in the field of pharmaceutical synthesis, can solve problems such as poor chemical selectivity, and achieves the effects of high selectivity, high yield, and wide popularization and application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0046] The techniques, features and effects of the present invention will be described below in conjunction with examples. Obviously, the described embodiments are only a part of the embodiments of the present invention, rather than all embodiments, based on the embodiments of the present invention, those skilled in the art without paying creative labor, all of which are obtained. The scope of the invention is protected.
[0047] The chemical reagents used in the following steps are all commercially available materials without further processing. Each step reaction yield is calculated as a column chromatography separation yield, and the steps are analyzed by the Qingdao silica gel, and the ethanol solution of sulfuric acid and phosphomolybolymolymented acid is heated after infiltration.
[0048] Nuclear magnetic spectrals were analyzed by Bruker Advance 500 (1H: 500MHz, 13C: 125MHz). The following abbreviations are used for splitting situations. S represents Singlet, D represents ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com