Process for preparing phenylhydrazine sulfate by continuous method
A sulfate, continuous process technology, applied in the preparation of hydrazine, organic chemistry, etc., can solve the problems of low yield, poor yield reproducibility, restricting equipment efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
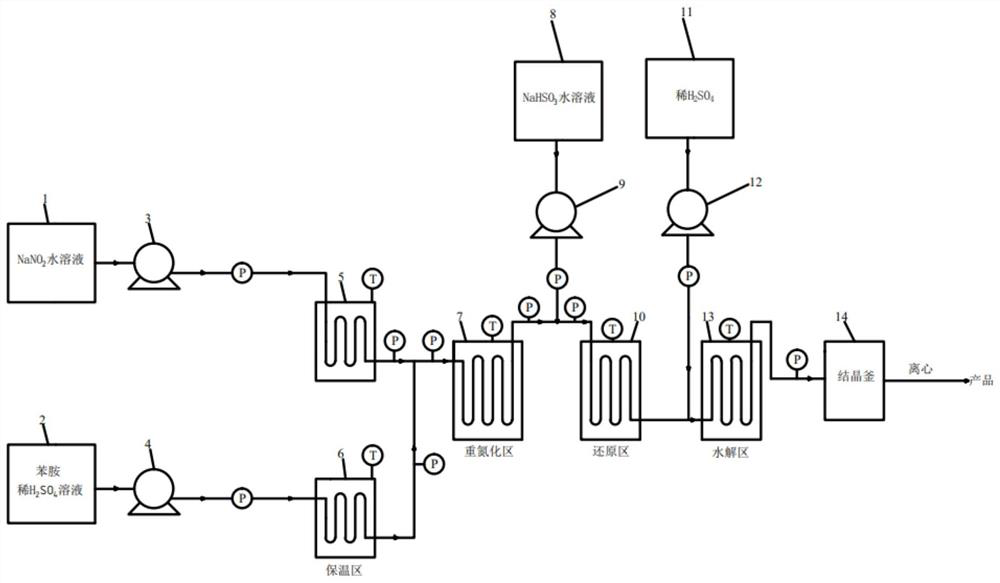
[0042] Embodiment 1, continuous method prepares aniline diazonium salt
[0043] Dissolve aniline in 15% sulfuric acid solution (aniline to sulfuric acid=1:1.2, molar ratio), 25% sodium nitrite solution according to aniline:NaNO 2= The ratio of 1:1.05 is pumped into the pipeline reactor by the metering pump respectively (see figure 1 diazotization zone), the temperature of the heat exchange medium (water bath or oil bath) is 25°C, and the residence time is 80s. The purity of the diazonium salt detected by sampling HPLC is about 97%, and the yield of the diazotization reaction is 96%. The obtained diazonium salt The solution is pumped directly into the next reaction.
[0044] The yield of aniline diazonium salt prepared by the method can be more than 95%.
Embodiment 2
[0045] Embodiment 2, continuous method prepares phenylhydrazine sulfate
[0046] The diazonium salt (the yield of diazotization reaction is estimated according to 95%), 20% NaHSO 3 Solution according to diazonium salt: NaHSO 3 = The ratio of 1:3 is pumped into the bamboo-shaped pulse tube reactor for continuous reaction (see figure 1 reduction zone), the temperature in this zone is set to T 1 ; After the material flows out, another metering pump is used to pump 40% dilute sulfuric acid into the reaction pipeline (see figure 1 hydrolysis zone) for hydrolysis, NaHSO in dilute sulfuric acid and reduction reaction 3 The molar weights are equal, and the temperature in the hydrolysis zone is set to T 2 , The material flowing out from the pipeline is directly passed into the crystallization kettle for cooling and crystallization, and then centrifugally filtered to obtain the product phenylhydrazine sulfate. The residence time in the reduction zone is set to t 1 , and the reside...
Embodiment 3
[0049] Embodiment 3, different NaHSO 3 Dosage
[0050] With reference to the method of embodiment 2, select the residence time and temperature condition of serial number 4 in table 1, change NaHSO 3 Dosage (referring to NaHSO 3 Consumption is the multiple of the amount of material of diazonium salt), other conditions are constant, and the result obtained is as shown in table 2:
[0051] Table 2. NaHSO 3 Dosage result
[0052]
[0053]
[0054] The result of table 2 shows: Yield increases with NaHSO 3 The increase of dosage increases, but when it exceeds 3 times, the range of increase is extremely limited.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com