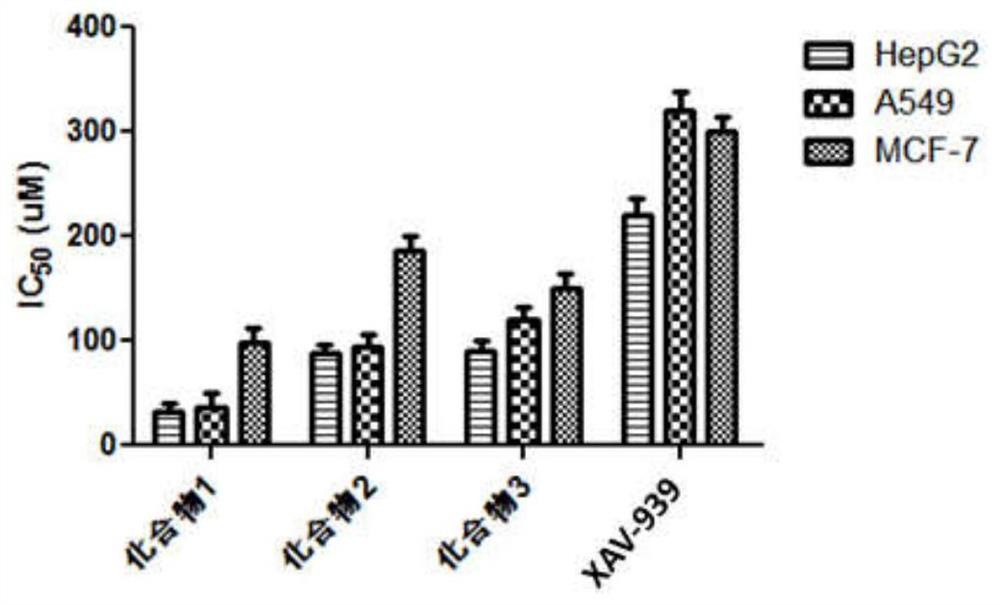
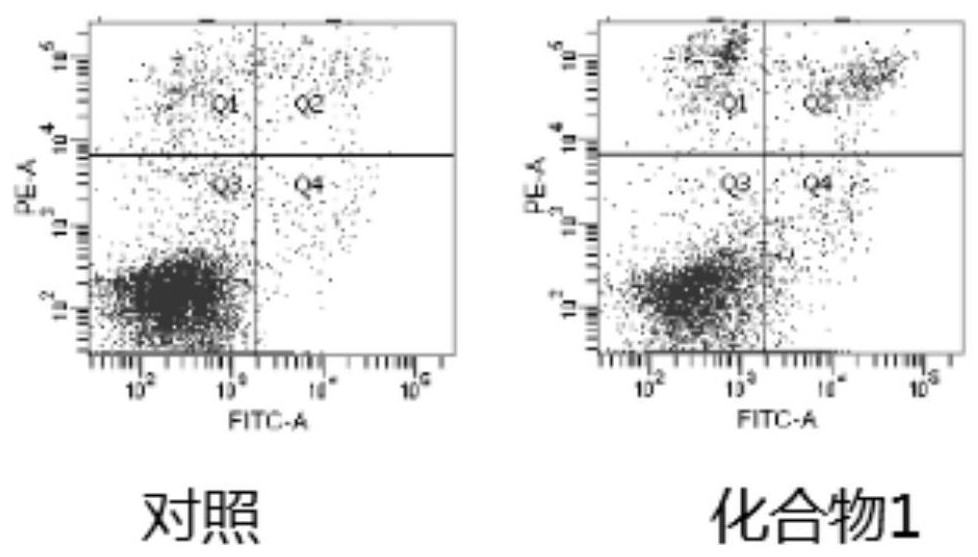
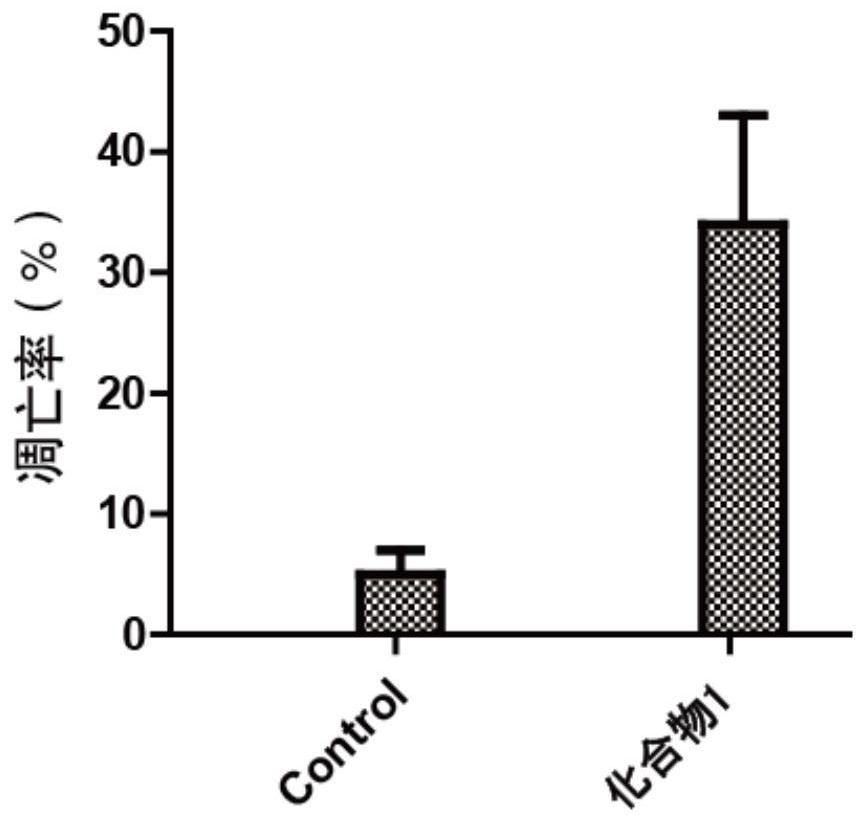
1, 3-dioxolane derivative compound as well as preparation method and application thereof
A technology of dioxolane and compounds, applied in the field of medicine, can solve unseen problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, the preparation of compound I
[0031] (1) Synthesis of 1-methoxy-1-phenylbut-3-en-2-ol (intermediate 1)
[0032]
[0033] To a solution of vinylmagnesium bromide (5.25 g, 40 mmol) in THF (30 mL) was added dropwise 2-methoxy-2-phenylacetaldehyde (5 g, 33.3 mmol) at 0°C. The mixture was stirred at 0 °C for 20 min, then washed with saturated NaHCO 3 (40 mL) solution to quench the reaction. The resulting solution was allowed to stand, and the layers were separated, the aqueous layer was extracted with ethyl acetate (3×20 mL), and the combined organic phases were dried over anhydrous sodium sulfate. The solvent was concentrated under reduced pressure followed by column chromatography on silica gel (20% EtOAc in hexanes as eluent) to give 1-methoxy-1-phenylbut-3-en-2-ol as a yellow oil , 5.28g, yield 88.9%.
[0034] 1 H-NMR (400MHz, CDCl 3 )δ:2.79(d,1H),3.16(s,3H),4.85(m,2H),5.29(d,2H),6.07(m,1H),7.32-7.42(m,5H). 13 C-NMR (125MHz, CDCl 3)δ: 57.42, 78...
Embodiment 2
[0050] Embodiment 2, the preparation of compound 2
[0051] (1), 3-(4-methyl-1,3-dioxolan-2-yl)-5-(methoxy(phenyl)methyl)dihydrofuran-2(3H)-one ( Synthesis of Intermediate 4)
[0052]
[0053] 5-(Methoxy(phenyl)methyl)furan-2(5H)-one (4.08g, 20mmol) and benzophenone (0.36g, 2mmol) were dissolved in 4-[1,3]-dioxo The solution in pentane (1.94 g, 22 mmol) was degassed for 1 h under a stream of argon. The mixture was then irradiated for 12 hours using a 450 W ACE glass medium pressure mercury lamp from a distance of 15 cm. pass 1 The progress of the reaction was observed by H-NMR. The reaction flask was kept in a water-cooled environment as the reaction mixture was degassed and throughout the irradiation time. The temperature of the cooling water is kept at around 0°C. Removal of the solvent under reduced pressure followed by purification by silica gel column chromatography (35% EtOAc in hexanes as eluent) afforded 3-(4-methyl-1,3-dioxolan-2-yl as a beige solid )-5-(met...
Embodiment 3
[0057] Embodiment 3, the preparation of compound 3
[0058] (1), 3-(4,5-Dimethyl-1,3-dioxolan-2-yl)-5-(methoxy(phenyl)methyl)dihydrofuran-2(3H) - Synthesis of Ketone (Intermediate 4)
[0059]
[0060] 5-(Methoxy(phenyl)methyl)furan-2(5H)-one (4.08g, 20mmol) and benzophenone (0.36g, 2mmol) were dissolved in 4,5-dimethyl-[1 A solution in ,3]-dioxolane (2.25 g, 22 mmol) was degassed under argon flow for 1 h. The mixture was then irradiated for 12 hours using a 450 W ACE glass medium pressure mercury lamp from a distance of 15 cm. pass 1 The progress of the reaction was observed by H-NMR. The reaction flask was kept in a water-cooled environment as the reaction mixture was degassed and throughout the irradiation time. The temperature of the cooling water is kept at around 0°C. After the reaction was complete, the solvent was removed under reduced pressure followed by purification by silica gel column chromatography (35% EtOAc in hexane as eluent) to give 3-(4,5-dimethyl-1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com