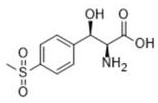
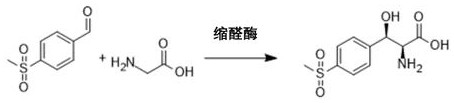
Method for synthesizing 2S,3R-p-methylsulfonephenylserine
A technology of thiamphenylphenylserine and p-thiamphenyl, applied in the field of synthesis of chiral 2S, 3R-p-thiamphenyl phenylserine, which can solve the problems of difficult reaction and difficult scale reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1. Preparation of chiral 2S, 3R-p-thiamphenicol phenylserine using transaldolase, aldehyde dehydrogenase and NAD oxidase
[0022] The reaction system (100 ml) in this example is composed of reaction solution, p-thymphenylbenzaldehyde, threonine, transaldolase, acetaldehyde condensing enzyme and NAD oxidase protein solution;
[0023] Among them, the reaction solution: 20 mM (pH 7.5) phosphate buffer solution; the concentration of p-thiamphenicyl benzaldehyde in the reaction system is 100 mM; transaldolase: the product number is GWTA08 commercial enzyme (purchased from Tianjin Industrial Microbiology Technology Co., Ltd. Company), the concentration in the reaction system is 1 mg / mL; acetaldehyde dehydrogenase, the product number is GWADR09 commercial enzyme (purchased from Tianjin Gong Microbiology Technology Co., Ltd.), the concentration in the reaction system is 0.5 mg / mL; NAD oxidase, the product number is GWAO16 commercial enzyme (purchased from Tianjin Gong M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com