Preparation method of 1-cyclohexyl-2-(morpholinoethyl) carbodiimide methyl p-toluenesulfonate
A technology of carbodiimide methyl and p-toluene sulfonate, applied in the preparation of sulfonate, organic chemistry, etc., can solve the problems of high toxicity of raw materials, difficult operation, and low overall repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of 1-cyclohexyl-2-(morpholine ethyl) carbodiimide methyl p-toluenesulfonate according to the embodiment of the present invention comprises:
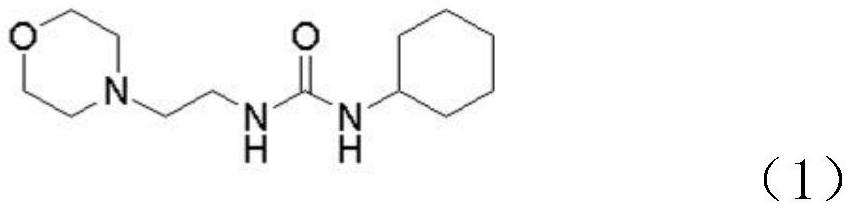
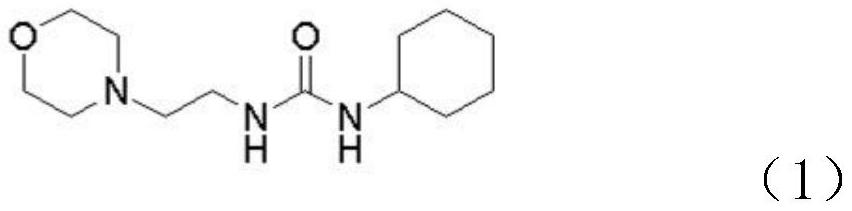
[0030] Step S1, adding N-(2-aminoethyl)morpholine to the first organic solvent, and then adding cyclohexyl isocyanate dropwise to generate intermediate 1, the chemical structure of intermediate 1 is shown in formula (1) .
[0031]
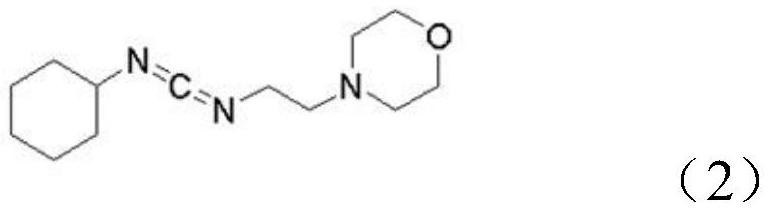
[0032] Specifically, its reaction formula is as shown in the following formula (3):
[0033]
[0034] Wherein, considering the solubility of each reaction raw material, the ease of recovery, the ease of operation, and the load on the environment, preferably, the first organic solvent is dichloromethane.
[0035] In addition, the molar ratio of N-(2-aminoethyl)morpholine to cyclohexyl isocyanate may preferably be 1:(1˜1.2). By adding cyclohexyl isocyanate slightly higher than the stoichiometric ratio, the reaction can be promoted and the yield can be improved.
[0036] In...
Embodiment 1
[0059] (1) Preparation of intermediate 1
[0060] Take 1L reaction flask and add N-(2-aminoethyl)morpholine (180g, 1.38mol, 1.0eq) and dichloromethane (540mL, 3P), dropwise add cyclohexyl isocyanate (173g, 1.3mol, 1.0eq), The temperature is controlled at 20-25° C., and the reaction is completed after being incubated for 2 hours. The reaction solution was concentrated to remove dichloromethane to obtain a crude product, which was then beaten with petroleum ether to obtain 343 g of CMC-1, with a melting point of 118.1-118.8° C. and a yield of 97.3%.
[0061] (2) Preparation of Intermediate 2
[0062] Take a 2L reaction flask and add triphenylphosphine (421.8g, 1.61mol, 1.2eq) into dichloromethane (1.3L, 5P), and add liquid bromine (257.6g, 1.61mol, 1.2eq) dropwise in an ice-water bath, Control the temperature at 10-20°C; continue to drop triethylamine (406g, 4.02mol, 3.0eq), control the temperature at 10-20°C; continue to dropwise add CMC-1 (343g, 1.34mol, 1.0eq), control the te...
Embodiment 2
[0067] (1) Preparation of Intermediate 1
[0068] Take 2L reaction flask and add N-(2-aminoethyl)morpholine (324g, 2.48mol, 1.0eq) and dichloromethane (1L, 3P), dropwise add cyclohexyl isocyanate (311.4g, 2.48mol, 1.0eq) , the temperature is controlled at 20-25° C., and the reaction is completed after insulated for 2 hours. The reaction solution was concentrated to remove dichloromethane to obtain a crude product, which was then beaten with petroleum ether to obtain 614 g of CMC-1, with a melting point of 118.3-119.1° C. and a yield of 96.8%.
[0069] (2) Preparation of Intermediate 2
[0070] Take a 5L reaction flask and add triphenylphosphine (756g, 2.89mol, 1.2eq) into dichloromethane (3L, 5P), and add liquid bromine (462.4g, 2.89mol, 1.2eq) dropwise in an ice-water bath to control the temperature At 10-20°C; continue to drop triethylamine (727.2g, 7.2mol, 3.0eq), control the temperature at 10-20°C; continue to dropwise add CMC-1 (614g, 2.4mol, 1.0eq), control the tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com