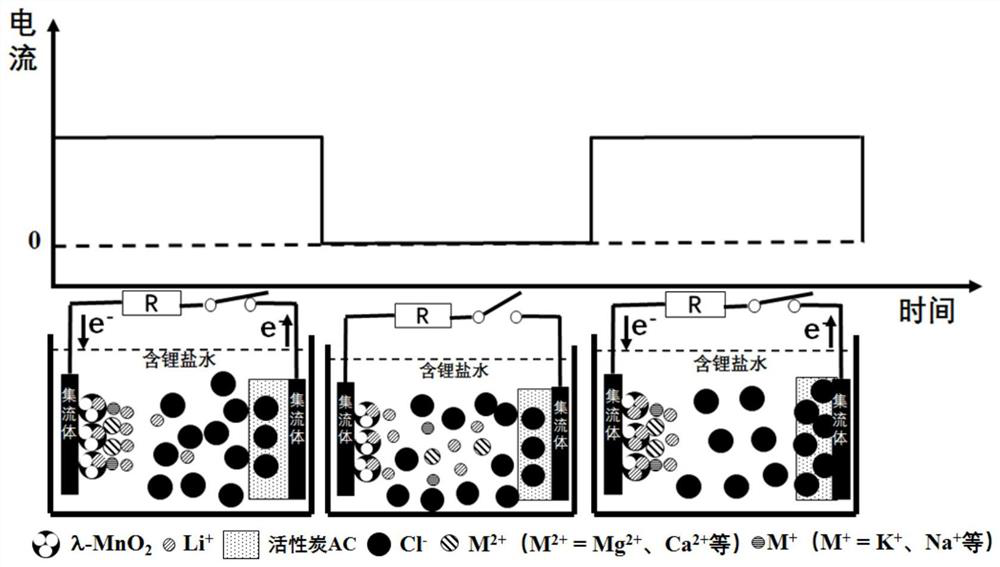
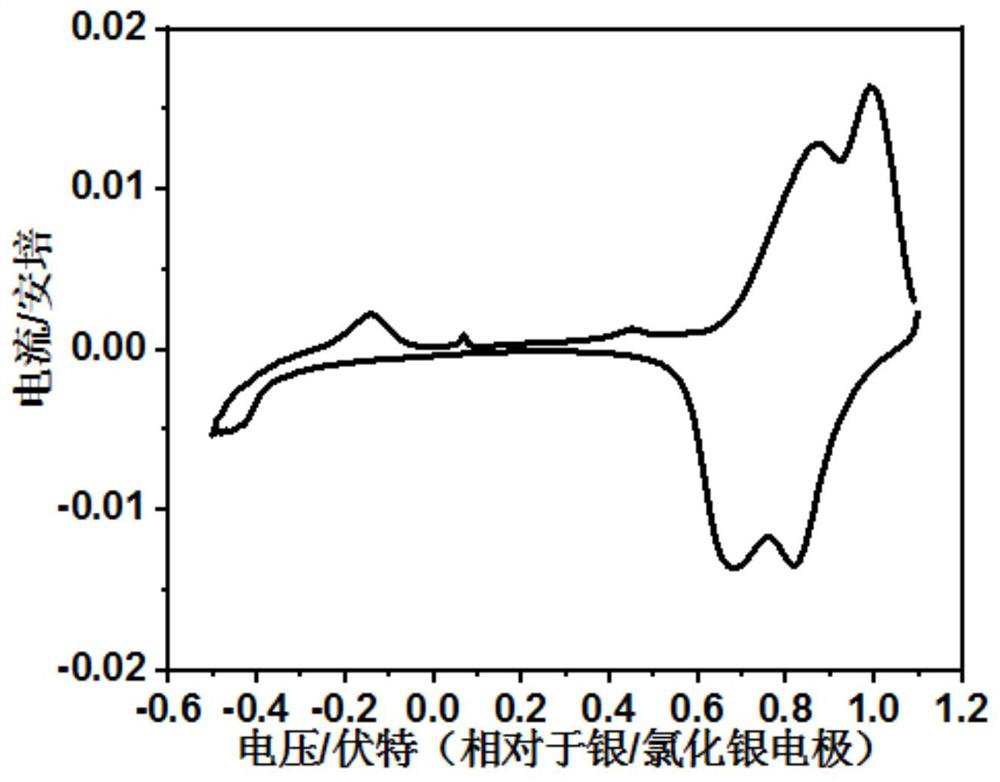
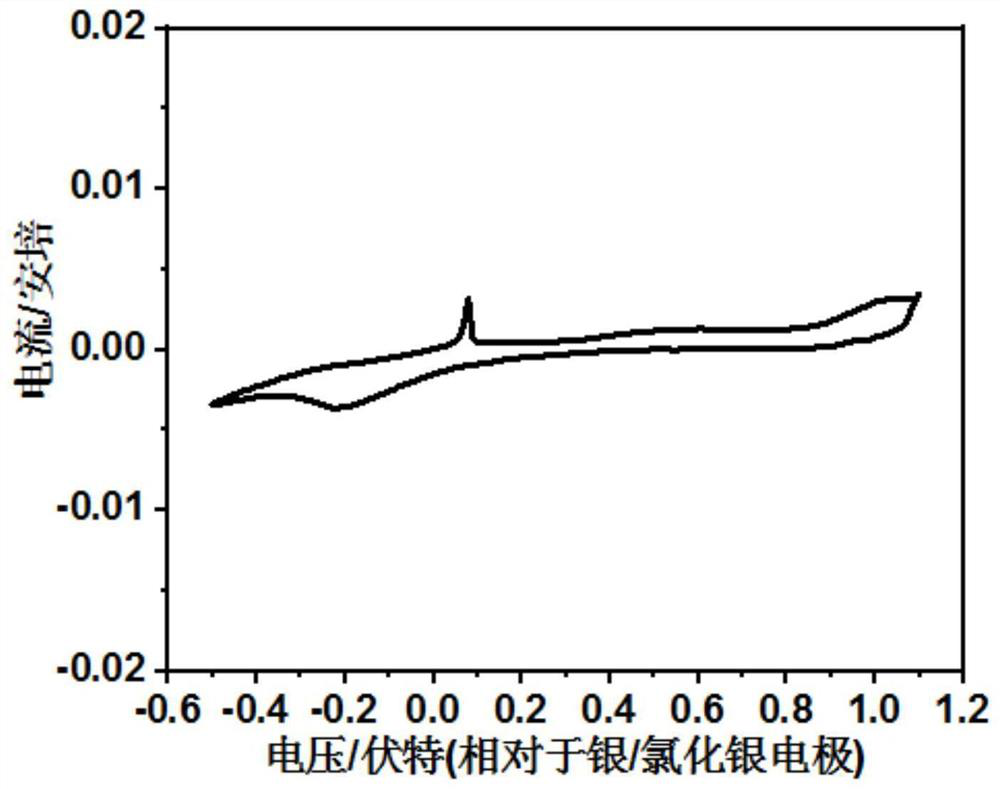
A method for electrochemically extracting lithium from brine to suppress the interference of coexisting cations
A technology of lithium ions and cations, applied in the field of high-efficiency extraction of lithium chloride, can solve the problems of increasing the exchange capacity of lithium ion sieves, complex liquid membrane preparation process, poor stability, etc., and achieves the suppression of the interference of coexisting cations, the process is simple, and the cost is low. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Weigh 16g of spinel lithium manganate LiMn 2 O 4 , 2g acetylene black conductive agent, 2g polyvinylidene fluoride PVDF binder and 98g N-methylpyrrolidone NMP, dissolve the weighed polyvinylidene fluoride PVDF binder in N-methylpyrrolidone NMP, then add Weighed spinel LiMn 2 O 4 and acetylene black conductive additive, stir to form a slurry, which is uniformly coated on the titanium mesh current collector, LiMn 2 O 4 The loading of 5mg / cm 2 , and then vacuum dried at 50 °C for 10 h to obtain LiMn 2 O 4 Electrode; with LiMn 2 O 4 The electrode is the working electrode, the titanium mesh is the counter electrode, the Ag / AgCl electrode is the reference electrode, and the LiCl solution with a concentration of 0.01mol / L is used as the electrolyte solution to construct an electrochemical reaction system. The electrode is connected for constant current charging, and the current density is 0.05A / g LiMn 2 O 4 , the charge cut-off voltage is 1.0V (vs. Ag / AgCl), and...
Embodiment 2
[0031] (1) Weigh 18g spinel type lithium manganate LiMn 2 O 4 , 1g acetylene black conductive agent, 1g polyvinylidene fluoride PVDF binder and 19g N-methylpyrrolidone NMP, dissolve the weighed polyvinylidene fluoride PVDF binder in N-methylpyrrolidone NMP, then add Weighed spinel LiMn 2 O 4and acetylene black conductive additive, stir to form a slurry, which is uniformly coated on the titanium mesh current collector, LiMn 2 O 4 The load is 10mg / cm 2 , and then vacuum dried at 100 °C for 5 h to obtain LiMn 2 O 4 Electrode; with LiMn 2 O 4 The electrode is the working electrode, the titanium mesh is the counter electrode, the Ag / AgCl electrode is the reference electrode, and the LiCl solution with a concentration of 0.05mol / L is used as the electrolyte solution to construct an electrochemical reaction system. The electrode is connected for constant current charging, and the current density is 0.01A / g LiMn 2 O 4 , the charge cut-off voltage is 0.9V (vs.Ag / AgCl), and t...
Embodiment 3
[0036] (1) Weigh 18g spinel type lithium manganate LiMn 2 O 4 , 1g acetylene black conductive agent, 1g polyvinylidene fluoride PVDF binder and 19g N-methylpyrrolidone NMP, dissolve the weighed polyvinylidene fluoride PVDF binder in N-methylpyrrolidone NMP, then add Weighed spinel LiMn 2 O 4 and acetylene black conductive additive, stir to form a slurry, which is uniformly coated on the titanium mesh current collector, LiMn 2 O 4 The loading of 7mg / cm 2 , and then vacuum dried at 80 °C for 7 h to obtain LiMn 2 O 4 Electrode; with LiMn 2 O 4 The electrode is the working electrode, the titanium mesh is the counter electrode, the Ag / AgCl electrode is the reference electrode, and the LiCl solution with a concentration of 0.1 mol / L is used as the electrolyte solution to construct an electrochemical reaction system. The electrode is connected for constant current charging with a current density of 0.1A / g LiMn 2 O 4 , the charge cut-off voltage is 1.1V (vs.Ag / AgCl), and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com