Synthesis method of benzofuran compound
A technology of benzofuran and synthesis method, applied in the field of organic chemical synthesis, can solve problems such as economical practicability limitation, increased operating cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
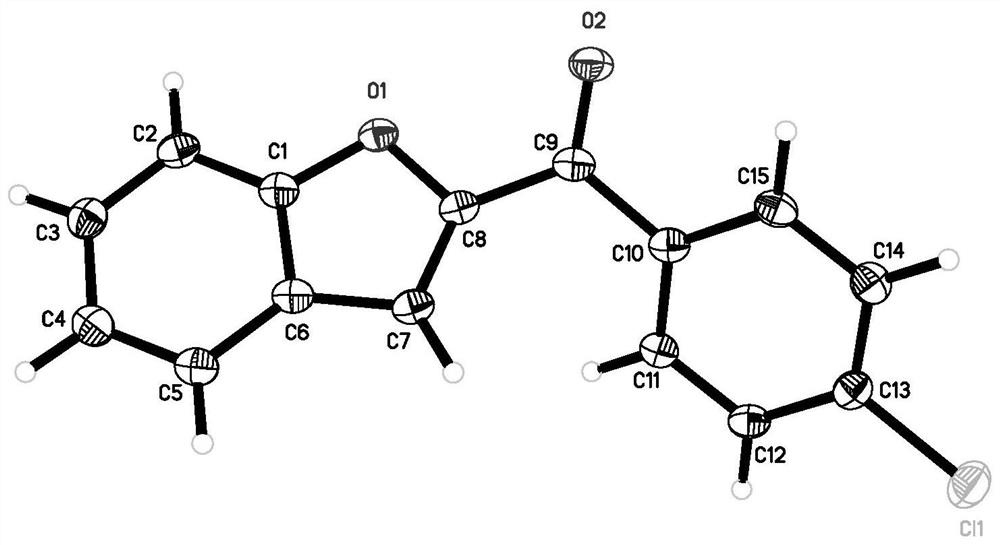
Image
Examples
Embodiment Construction
[0035] In order to make the object, technical solution and advantages of the present invention clearer, the present invention will be further described in detail below in conjunction with the accompanying drawings. What is disclosed below is only a preferred embodiment of the present invention, which certainly cannot limit the scope of the present invention. Therefore, equivalent changes made according to the claims of the present invention still fall within the scope of the present invention.
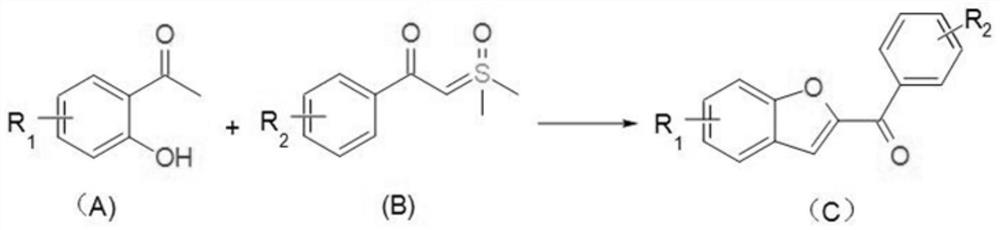
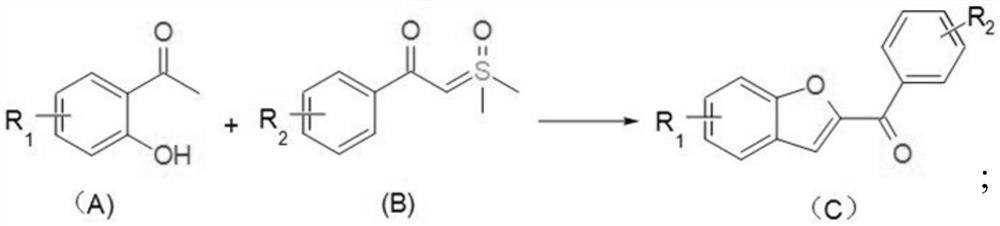
[0036] The invention provides a kind of synthetic method of benzofuran compound, and its chemical reaction formula is as follows:
[0037]
[0038] In the chemical reaction formula, the compound shown in (A) and the compound shown in (B) react in an organic solvent under the effect of a transition metal catalyst and a base;
[0039] The transition metal catalyst is iron phthalocyanine;
[0040] Described alkali is one or more in potassium carbonate, sodium carbonate, cesium carbona...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com