Quinone reductase 2 inhibitors for use as neuroprotective agents
A nerve injury and alkyl technology, which can be used in nervous system diseases, medical preparations containing active ingredients, pharmaceutical formulas, etc., and can solve problems such as limited efficacy and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0213] Example 1: Development of non-lysosomal aminoquinoline inhibitors of QR2
[0214] Chloroquine and hydroxychloroquine are lysosomal drugs that preferentially accumulate in cellular lysosomes. Their structure is:
[0215]
[0216] For chloroquine, the pKa of the tertiary amine nitrogen is 10.32, and the pKa of the quinoline nitrogen is 7.29. At pH 4 to 5.5 in acidic lysosomes, almost 100% of chloroquine is thus doubly protonated, giving the molecule a 2 + The charge makes the molecule strongly hydrophilic, membrane impermeable, and thus trapped in acidic organelles.
[0217] This trapping phenomenon can be addressed quantitatively by measuring the octanol-water partition coefficient logD of the drug, which describes the relative partition properties of all forms of the compound at different pH. For a given pH, compounds with a positive logD are relatively lipophilic and more membrane permeable, while compounds with a negative logD are hydrophilic and less membrane p...
Embodiment 2
[0244] Embodiment 2: Synthesis and characterization of 7-chlorinated compounds
[0245]
[0246] A suspension of 4,7-dichloroquinoline (2.0 g, 10.2 mmol) in aqueous methylamine (40% 20 mL 260 mmol, 26 eq.) was placed in a microwave vessel at 90 °C (initial power set to 150 W ) heated for 2 hours. By TLC (2% in CH 2 Cl 2 Analysis of the reaction mixture showed complete consumption of starting material. with H 2 O (100 mL) diluted the reaction mixture and collected the insolubles in vacuo. filter cake with H 2 O washed and dried in vacuo to give the pure product as a white microcrystalline solid (1.8 g, 92%). 1 H NMR (DMSO- d 6 , 300 MHz) δ 8.40 (d, J = 5.1 Hz, 1H), 8.16 (d, J =9.0 Hz, 1H), 7.77 (s, 1H), 6.38 (d, J = 5.4 Hz, 1H), 2.86 ( d, J = 5.4 Hz,3H). ESIMS: m / z = 193 [(M+H) + ].
[0247]
[0248] General procedure for 7-substituted-4-(pyridin-3-yl)-methylaminoquinolines. A mixture of 7-substituted-4-chloroquinoline (5.1mmol), 3-aminomethylpyridine (0.70...
Embodiment 3
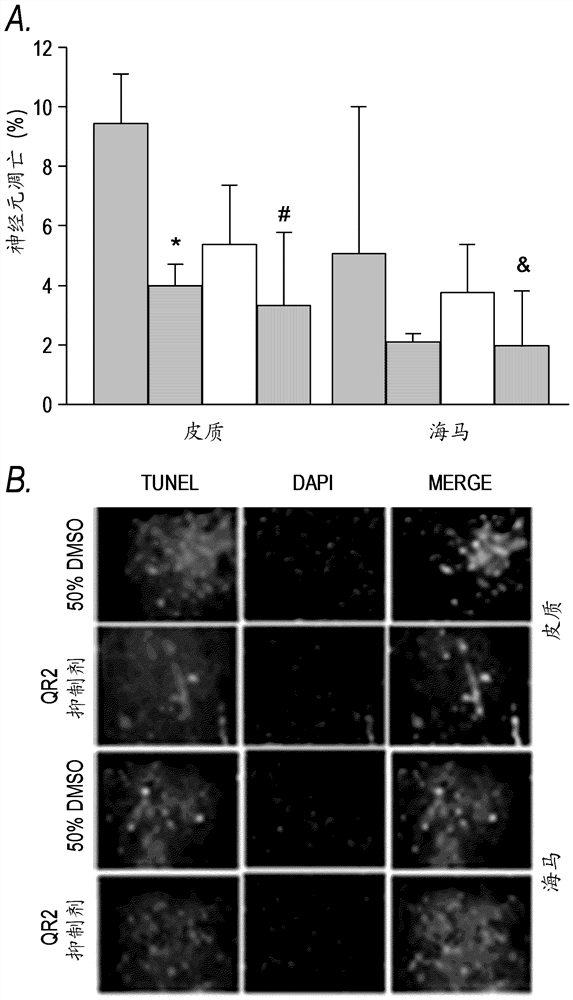
[0251] Example 3: Evidence for the protective effect of QR2 inhibition in cerebral infarction and the therapeutic effect of non-lysosomal QR2 inhibitors.
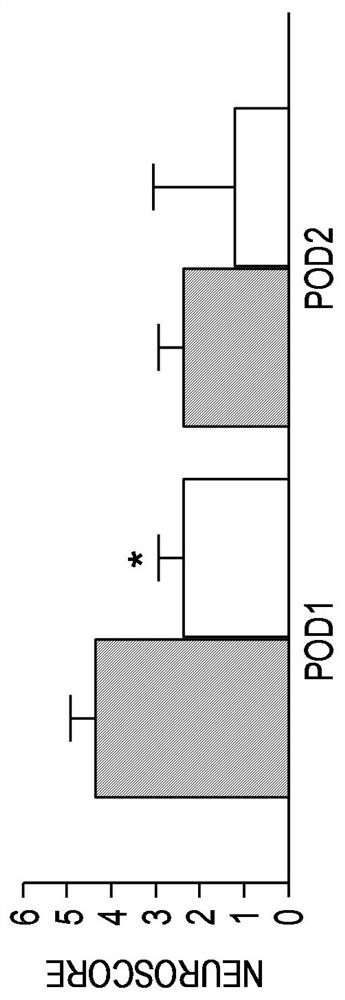
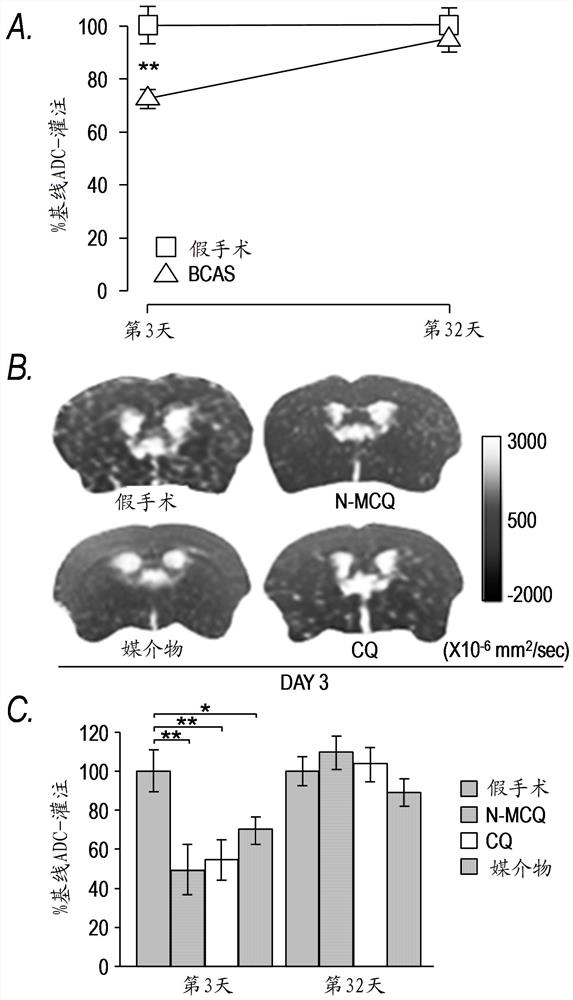
[0252] The neuroprotective efficacy of chloroquine (CQ) was demonstrated in a mouse model of transient middle cerebral artery (MCA) occlusion. Histological assessment at autopsy at 72 hours showed that a single intraperitoneal (i.p.) administration of CQ (25 mg / kg) 90 minutes after the onset of ischemia resulted in a 55% reduction in total stroke volume as measured by diffusion-weighted magnetic resonance imaging (DW- As measured by MRI, within 4 to 24 hours, there was a corresponding reduction in stroke development with improvements in neurological scores and motor function, as measured by Figures 3A-3C shown.
[0253] The non-lysosomal QR2-selective 4-aminoquinoline, 7-chloro-N-methylquinolin-4-amine (7C-4MAQ, Example compound F shown above) was also tested in the same model. Neuroprotective effect. In this animal mod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com