Unsaturated spiro orthocarbonate expanding monomer as well as synthesis method and application thereof
A spirocyclic orthocarbonate and expanding monomer technology, applied in adhesive additives, non-polymer adhesive additives, organic chemistry, etc., can solve problems such as being inferior to epoxy resins, limiting wide application, reducing shrinkage rate, etc. , to achieve the effect of improving adhesion, reducing resin shrinkage and reducing internal stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In the present embodiment, the synthetic method of unsaturated spiro ring orthocarbonate expansion monomer comprises the following steps:
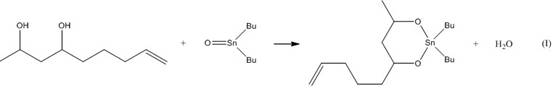
[0033] (1) Weigh 6,8-dihydroxy-1-nonene, di-n-butyltin oxide and toluene (6,8-dihydroxy-1-nonene 0.1mol, di-n-butyltin oxide 0.1mol, toluene 150ml), added to the reaction vessel and stirred, and reacted at 150±2°C for 12 hours or until anhydrous was formed (in this step (1), the reaction vessel was a three-necked flask placed in an oil bath, and 6, After adding 8-dihydroxy-1-nonene, di-n-butyltin oxide and toluene into the three-necked flask, install a water separator and a reflux condenser, install a calcium chloride drying tube on the reflux condenser, and then carry out the reaction) ;
[0034] This step reacts by reaction formula (I), and continuously removes the water that generates in the reaction process;
[0035]
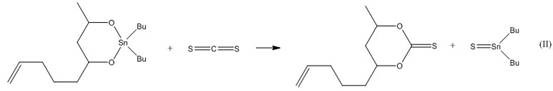
[0036] (2) Cool the reaction liquid in the reaction vessel to room temperature, then add carbon disulfide ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| bending strength | aaaaa | aaaaa |
| shear strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com