Simple and efficient lentivirus cryopreservation liquid and application thereof
A technology of lentivirus and cryopreservation solution, which is applied in the field of chimeric antigen receptor lentivirus cryopreservation solution, which can solve the problems of loss of lentivirus infection efficiency and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: CD19 CAR lentiviral vector construction
[0021] FMC63 scFv fragment SEQ ID NO: 1, and the second-generation CAR hinge region, transmembrane region, and intracellular region sequence SEQ ID NO: 2 corresponding to the signal peptide, were synthesized by BGI. After obtaining the two synthetic genes, construct the vector of CD19 CAR. First, amplify by PCR and add restriction sites at the head and tail of the two sequences, and then digest and ligate to obtain SEQ ID NO: 3, which will be slow The viral vector pCDH-EF1a-T2A-puro and SEQ ID NO: 3 were double-enzymatically digested and ligated with T4 DNA ligase respectively, transformed into competent cells, single clones were picked, and after the bacterial liquid was sequenced correctly, the plasmid was extracted to obtain the correct sequence Lentiviral vector pCDH-EF1a-CD19 CAR plasmid.
Embodiment 2
[0022] Example 2: Preparation of CD19 CAR lentivirus
[0023] After mixing the lentiviral packaging plasmid mixture (including PSPAX2 and VSVG) and the pCDH-EF1a-CD19 CAR plasmid in a pre-optimized ratio, add transfection-assisting reagents and incubate at room temperature for 30 minutes. The transfection mixture was then slowly added to 293T cells (purchased from ATCC). After 1-3 days, the medium supernatant was collected to obtain the crude lentivirus solution. A total of 180 ml of the supernatant was collected after centrifugation at 500 g for 10 min at 4°C. Divide 180 ml into 6 tubes on average, and place each 30 ml tube in an ultra-high-speed centrifuge tube. After balancing, ultra-centrifuge at 20,000 g for 2 hours to concentrate the lentivirus.
Embodiment 3
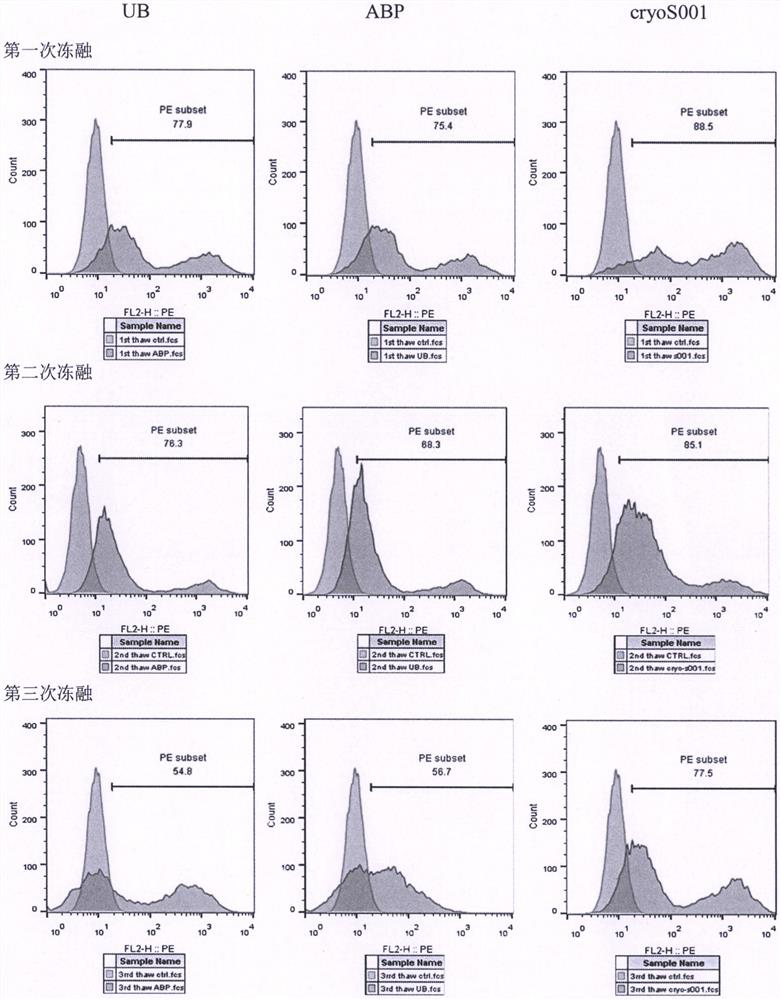
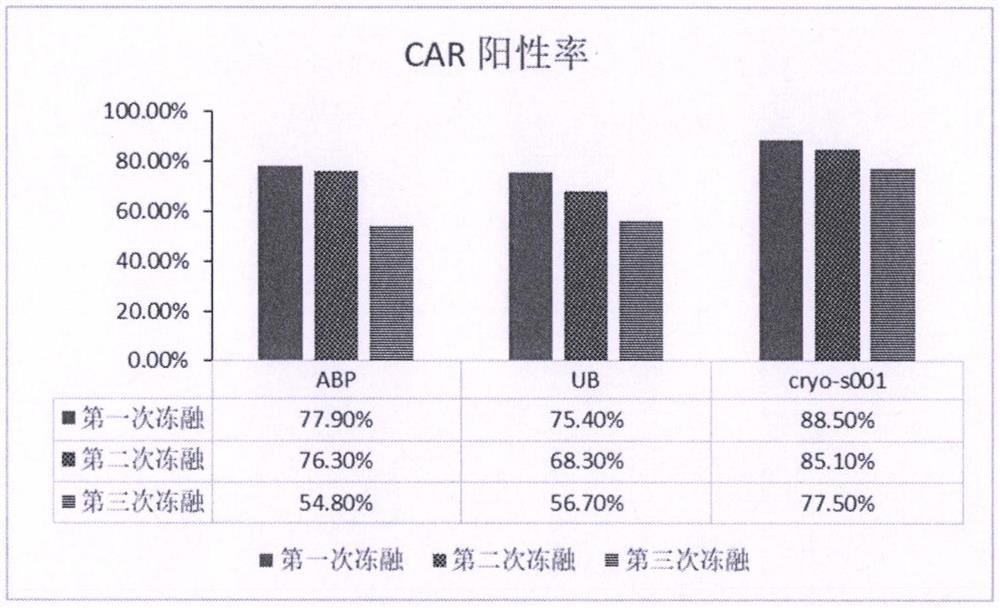
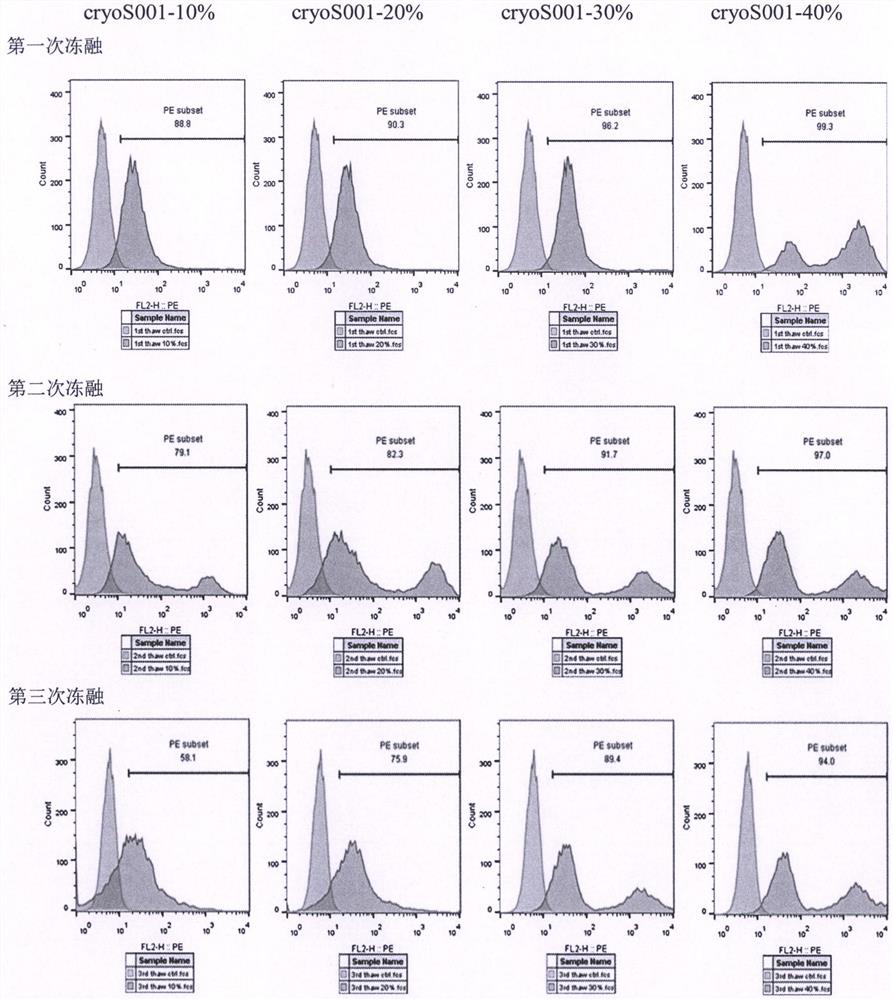
[0024] Example 3: Pre-experiment of lentiviral cryopreservation solution
[0025] In this example, the lentivirus cryopreservation solution of the present invention (hereinafter referred to as cryoS001) and two commercial lentivirus cryopreservation solutions (brand 1, Yumeibo, article number UR40202, hereinafter code-named UB; brand 2, Andy FUNO, Cat. No. D022 hereinafter code-named ABP) compared the protection efficiency of CD19 CAR lentivirus to clarify the effectiveness of the lentivirus cryopreservation solution.
[0026] After the ultracentrifugation of the aliquoted lentivirus in the above Example 2, carefully discard the supernatant, and resuspend the lentiviral particles with the pre-cooled lentiviral freezing solution: put 60 ml of lentivirus in two ultracentrifugation tubes After concentrating the lentivirus stock solution, discard the supernatant, resuspend each tube with 0.5 ml of UB or ABP, or cryoS001, that is, divide the ultra-concentrated 180 ml lentivirus sto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com