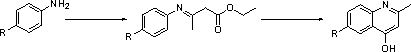
Synthesis process of quinoline medical intermediate
A synthesis process and intermediate technology, which is applied in the field of synthesis process of quinoline series pharmaceutical intermediates, which can solve the problems of scorched products, high temperature, and isomers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: The present invention provides a synthesis process of quinoline series pharmaceutical intermediates. Step 1: When the room temperature is 20°C, dissolve 8kg of SM in 56L of ethanol, add 8.5kg of ethyl acetoacetate, and add 12.5kg of anhydrous magnesium sulfate , add 280mL of acetic acid, heat up to reflux at 80°C-85°C, and finish the reaction after 18 hours of reaction;
[0020] Step 2: Cool the solution to room temperature, filter, drain the filter cake, rinse with ethanol, and concentrate the filtrate to dryness to obtain a reddish-brown liquid;
[0021] Step 3: When the red-brown liquid in step 2 is heated up to 70°C, pour 75% sulfuric acid into a 50L reaction kettle, and when the temperature rises to 80°C, slowly drop 8kgSM into the reaction bottle;
[0022] Step 4: Stir for 30 minutes after the addition is complete, detect that there is no raw material, add 10L of water dropwise, and then process after the addition;
[0023] Step 5: Cool the solution...
Embodiment 2
[0028] Embodiment 2: The present invention provides a quinoline series pharmaceutical intermediate synthesis process, step 1: when the room temperature is 20°C, dissolve 7kgSM in 56L ethanol, add 7.5kg ethyl acetoacetate, add 11.5kg anhydrous magnesium sulfate , add 250mL acetic acid, heat up to reflux at 75°C-85°C, and finish the reaction after 16 hours of reaction;
[0029] Step 2: Cool the solution to room temperature, filter, drain the filter cake, rinse with ethanol, and concentrate the filtrate to dryness to obtain a reddish-brown liquid;
[0030] Step 3: When the reddish-brown liquid in step 2 is heated up to 60°C, pour 65% sulfuric acid into a 50L reaction kettle, and when the temperature rises to 70°C, slowly drop 7kgSM into the reaction flask;
[0031] Step 4: Stir for 30 minutes after the addition is complete, detect that there is no raw material, add 10L of water dropwise, and then process after the addition;
[0032] Step 5: Cool the solution to room temperature ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap