Undiscriminated protein thermal stability analysis method
An analysis method and thermal stability technology, applied in the direction of analyzing materials, material inspection products, measuring devices, etc., can solve problems such as no revelation, achieve wide coverage, avoid experimental errors, and non-discriminatory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The new method of protein thermal stability analysis based on non-discrimination is used in the research of the target protein of Stauroporine (stauroporine):
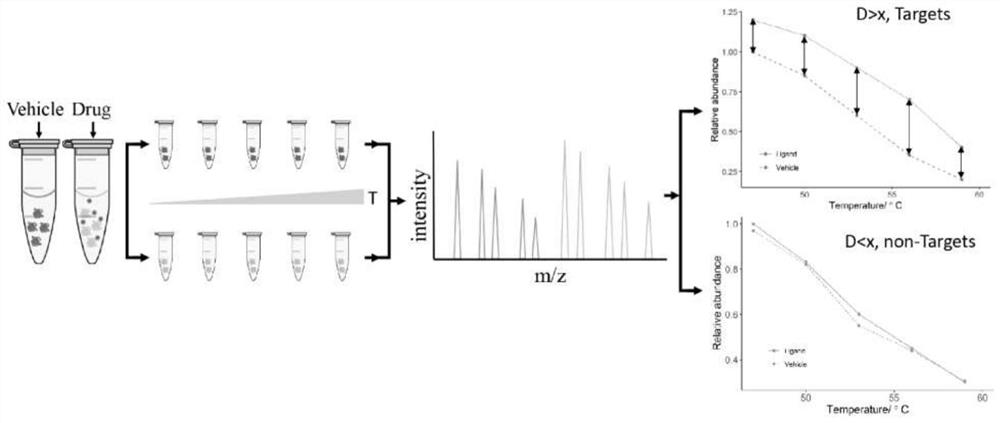
[0040] (1) Take 1 plate of K562 cell samples, resuspend in 600 μL PBS buffer solution, add protease inhibitors (AEBSF, aprotinin, bestatin, E-64, leupeptin) with a final volume concentration of 1% dimethyl sulfoxide (DMSO) solution of a mixture of peptide and pepstatin A), after repeated freezing and thawing through liquid nitrogen three times, centrifuge at 20,000g for 10min at 4°C, and collect the supernatant sample;
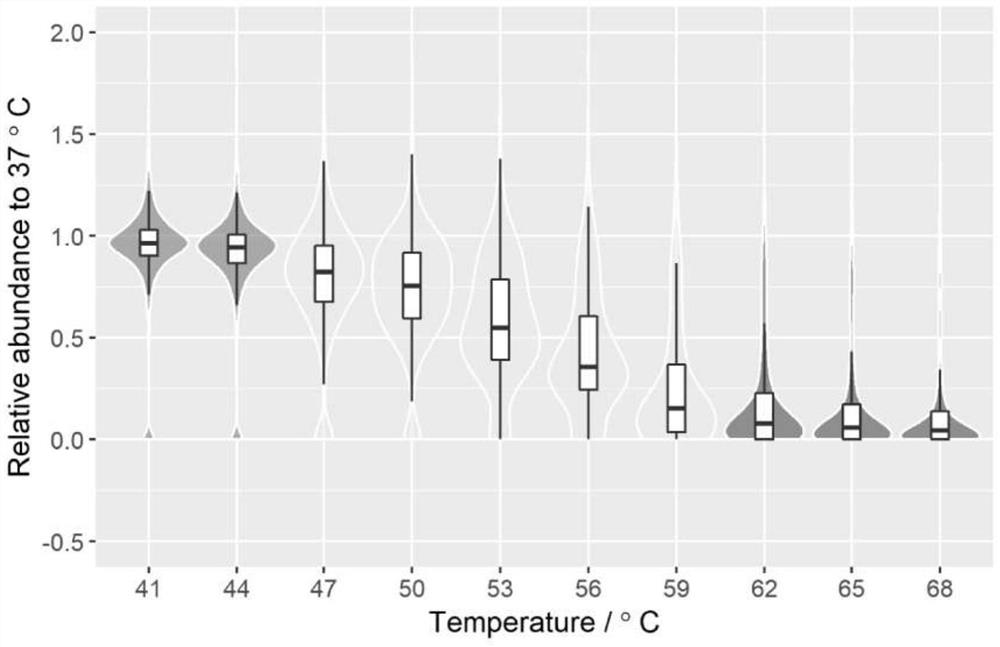
[0041] (2) Take 50 μL of the above protein samples in eleven new EP tubes at 37°C, 41°C, 44°C, 47°C, 50°C, 53°C, 56°C, 59°C, 62°C, 65°C, Heat at 68°C for 3 minutes, then cool at room temperature for 2 minutes, and then centrifuge at 4°C and 20,000g centrifugal force for 10 minutes. Take 30 μL of the supernatant in each EP tube and transfer them to eleven new EP tubes for trypsin (trypsin) diges...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap