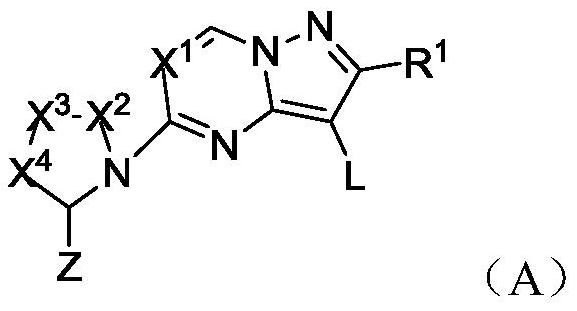
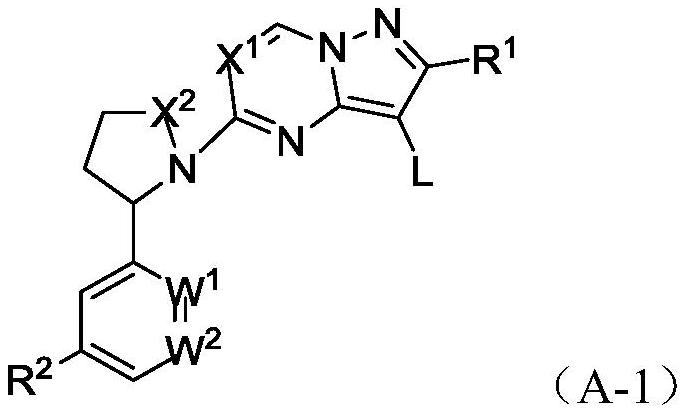
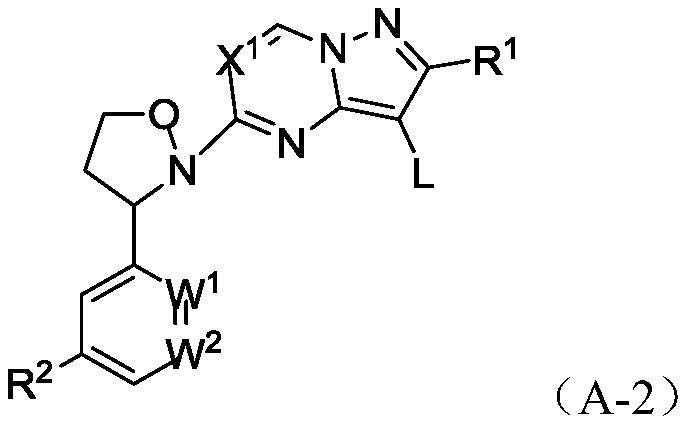
Heteroaryl fused ring compound as well as preparation method and application thereof
A technology of heteroaryl and compound, applied in the application field of heteroaryl condensed ring compound and TRK inhibitor, can solve the problem of drug resistance, and achieve the effect of low inhibitory dose, good inhibitory effect and high inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0137] Preparation Example 1: Preparation of (R)-5-fluoro-2-methoxy-3-(pyrrolidin-2-yl)pyridine
[0138]
[0139] The intermediate of Preparation Example 1 can be prepared by various preparation methods disclosed in the prior art, for example, by the method described in the PCT patent with the publication number WO2011146336 A1 or the US patent with the publication number US20160168156 A1.
preparation example 2
[0140] Preparation Example 2: Preparation of 5-chloro[1,5-a]pyrimidine-3-carbaldehyde
[0141]
[0142] The intermediate of this preparation example 2 can be prepared by various preparation methods disclosed in the prior art, for example, by the method described in the US patent publication number US20140234254 A1 or the Chinese patent publication number CN103965199A.
preparation example 3
[0143] Preparation Example 3: Preparation of 1-((2-(trimethylsilyl)ethoxy)methyl)-1H-imidazole
[0144]
[0145] Dissolve imidazole (6.8g) in tetrahydrofuran (100mL), add sodium hydride (36.0g) in batches while maintaining 0°C under nitrogen protection, after the addition is complete, stir the system at room temperature for 1 hour, then add chloromethyltrimethylsilyl ethyl Diethyl ether (20.0 g) was dissolved in tetrahydrofuran and slowly added to the reaction system. The system was kept at room temperature for overnight reaction. TLC and LCMS showed that the raw materials were completely consumed. The reaction system was poured into water, extracted twice with ethyl acetate, and the resulting filtrate was dried over anhydrous sodium sulfate and concentrated. The crude product was purified by column chromatography (petroleum ether / ethyl acetate=50:1 (V:V)) to obtain 7.7 g of the title compound.
[0146] MS(ESI)m / z(M+H) + = 199.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com