Preparation method of SGLTs inhibitor and key intermediate thereof
A system and compound technology, applied in the preparation of sugar derivatives, chemical instruments and methods, organic chemistry, etc., can solve problems such as unfavorable industrial production, achieve the problem of drug accessibility, strong operability, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
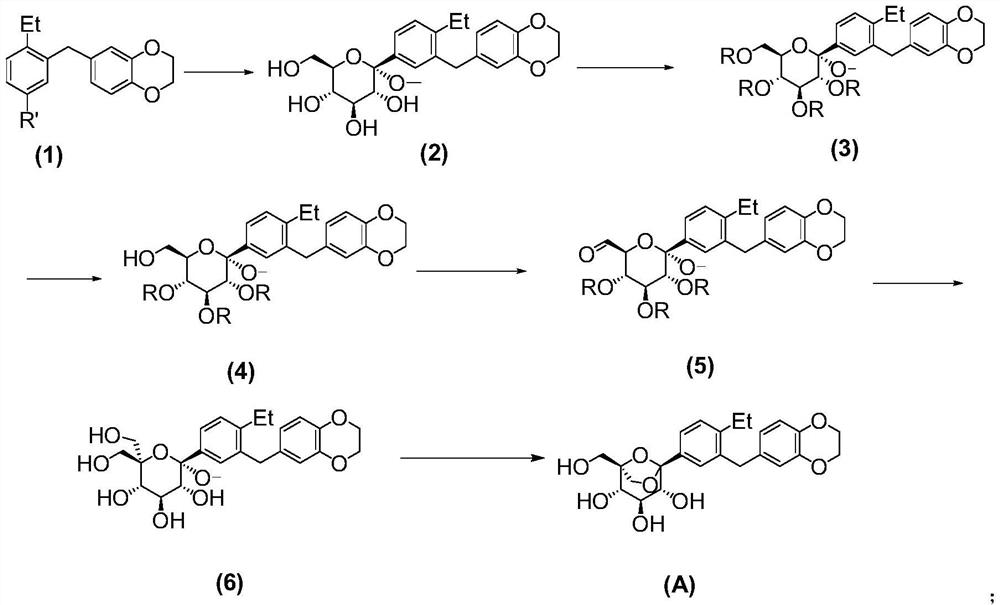
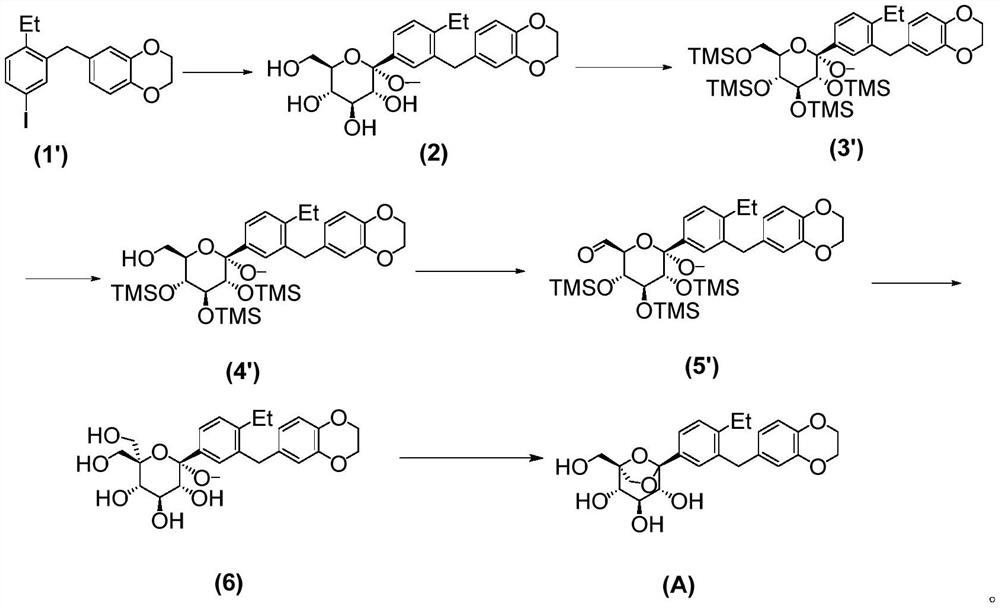
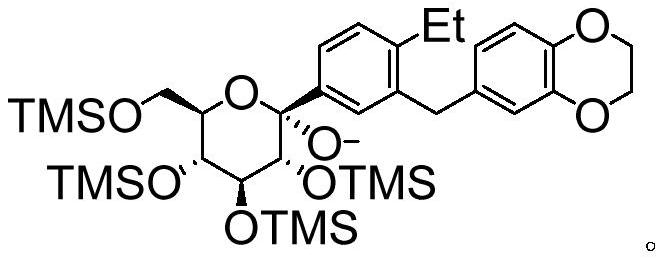
[0043] The inventors of the present application have developed a preparation method of the SGLTs inhibitor with the compound structure of formula (A) after extensive and in-depth research. The preparation method uses 6-(2-ethyl-5-iodobenzyl)-2,3-dihydrobenzo[b][1,4]dioxin as a raw material, and uses a silyl group as a protecting group , prepared by a six-step reaction. The preparation method of the invention is simple to operate, has high yields in each step, and stable process, can adapt to the needs of industrial production, solves the problem of drug accessibility, and is beneficial to accelerate the clinical development and drug marketing of SGLTs inhibitors. On this basis, the present invention has been accomplished.
[0044]The present invention will be described in further detail and completely below in conjunction with the examples, but it is by no means limiting the present invention, and the present invention is not limited only to the content of the examples.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com