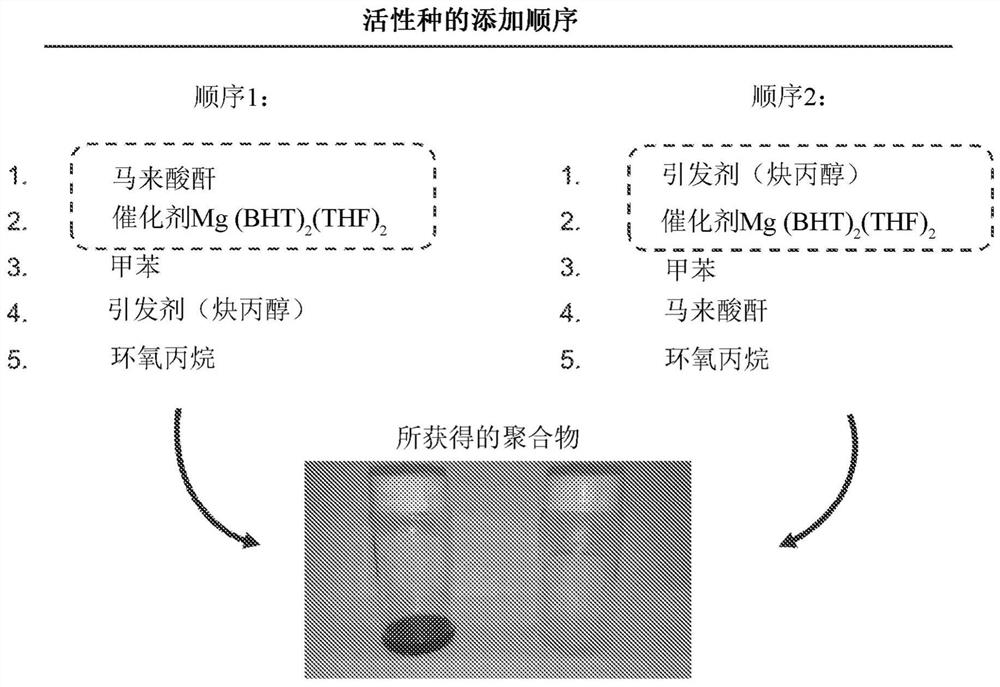
Poly(propylene fumarate)-based copolymers for 3D printing applications
A 3D, polymer technology, applied in 3D object support structure, processing and manufacturing, manufacturing tools, etc., can solve problems such as limited production volume, high light source, 3D printing failure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0148] The following examples are provided to more fully illustrate the invention, but should not be construed as limiting the scope of the invention. Further, while some of the examples may contain conclusions about the manner in which the invention may function, the inventors do not wish to be bound by these conclusions, but merely present them as possible explanations. Also, unless indicated by use of the past tense, the representation of an example does not imply that an experiment or procedure has or has not been performed or that a result has or was not actually obtained. Efforts have been made to ensure accuracy with respect to numbers used (eg, amounts, temperature) but some experimental errors and deviations may be present. Unless indicated otherwise, parts are parts by weight, molecular weight is weight average molecular weight, temperature is in degrees Centigrade, and pressure is at or near atmospheric.
[0149] Material
[0150] All materials were purchased from...
example 1
[0162] Copolymer synthesis of DP 20 polymer with 5 mol% succinic anhydride feed ratio
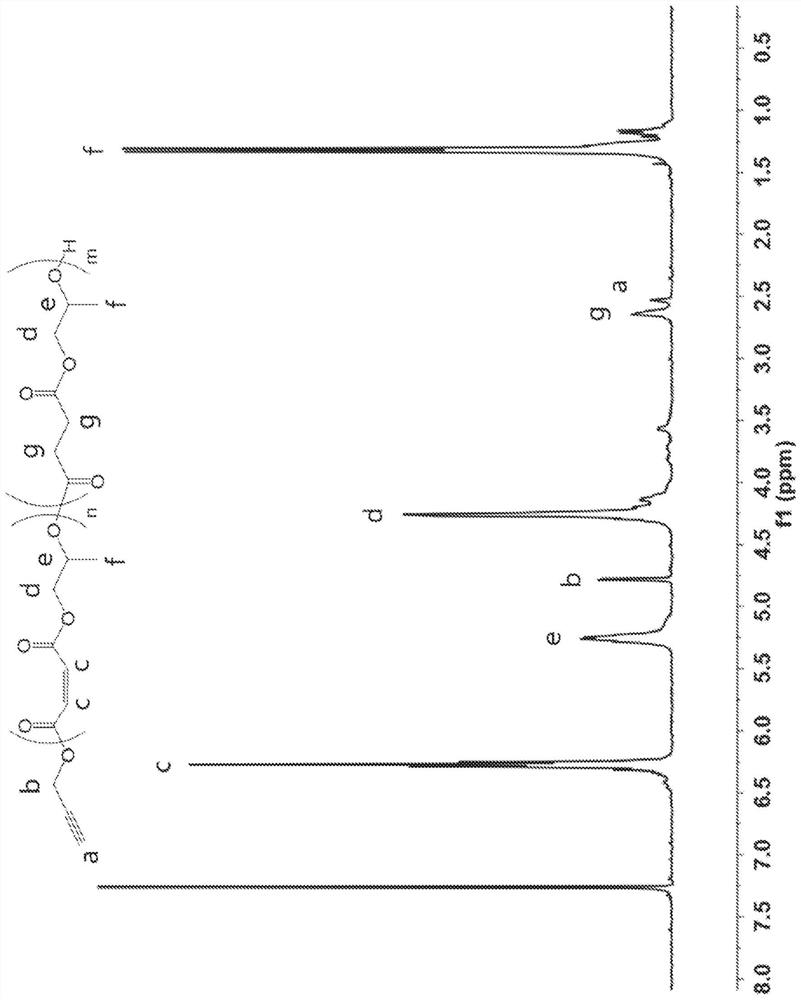
[0163] A DP 20 polymer having a 5 mol% succinic anhydride feed ratio was synthesized following the same procedure shown in Comparative Example 1, except that, instead of 2.80 g maleic anhydride, 2.664 g maleic anhydride and 143.2 mg succinic anhydride were added (i.e. , 2.664g maleic anhydride, 0.143g succinic anhydride, 2mL propylene oxide, 148.6uL benzyl alcohol, 174mg Mg(BHT) 2 (THF) 2 ), 4.42 g (99.0%) of copolymer were recovered. The resulting copolymer passed 1 H NMR characterization ((300MHz, 298K, CDCl 3 ): 7.36(s, 5.0H, Ar), 6.36-6.18(m, 49.0H, C=OCHCHC=O), 5.35-5.14(m, 26.9H, CH 2 CHCH 3 O), 4.37-4.00 (m, 52.7H, OCH 2 CHCH 3 ),2.67-2.55(m,2.7H,C=OCH 2 CH 2 C=O), 1.40-1.08(m, 77.9, CHCH 3 )). DP 20 copolymer with 5mol% succinic anhydride 1 H NMR spectrum as Figure 14 shown. The degree of polymerization was confirmed by NMR, and the number average molecular weight (M)...
example 2
[0165] Polymerization with DP 20 and 10 mol% succinic anhydride feed ratio
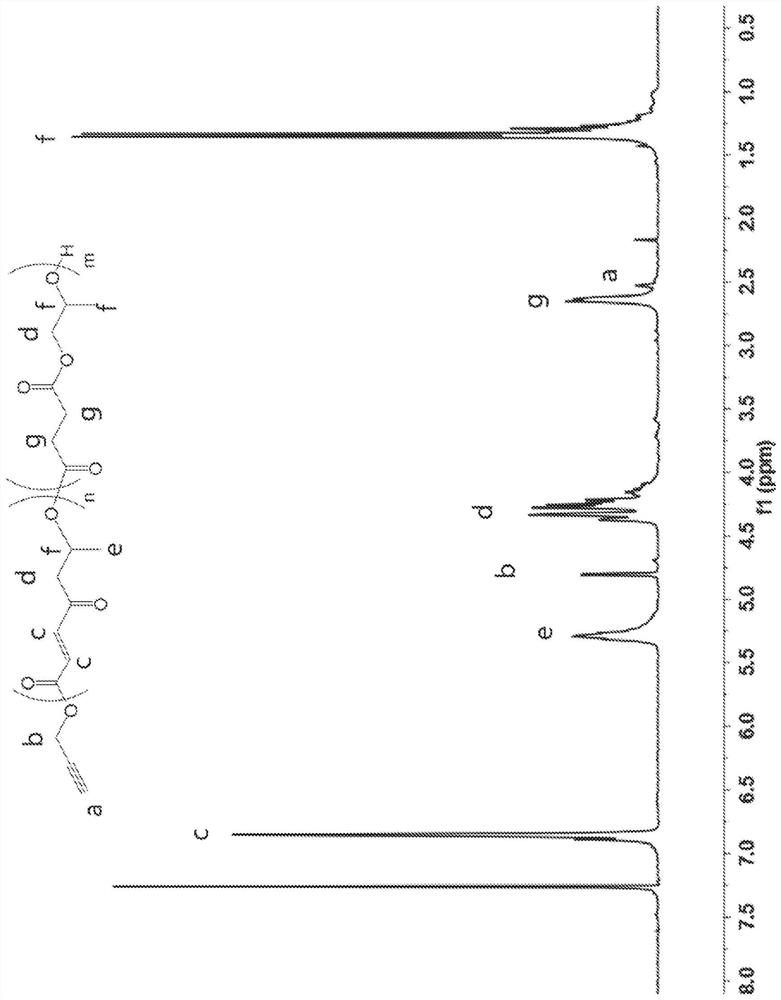
[0166] A DP 20 copolymer having a 10 mol% succinic anhydride feed ratio was synthesized following the procedure shown in Comparative Example 1, except that, instead of 2.80 g maleic anhydride, 2.524 g maleic anhydride and 286 mg succinic anhydride were used (i.e., using 2.524g maleic anhydride, 0.286g succinic anhydride, 2mL propylene oxide, 148.6uL benzyl alcohol, 174mg Mg(BHT) 2 (THF) 2 ). 4.46 g (99.8%) of copolymer were recovered. The resulting copolymer passed 1 H NMR characterization ((300MHz, 298K, CDCl 3 ): 7.36(s, 5.0H, Ar), 6.36-6.18(m, 43.0H, C=OCHCHC=0), 5.35-5.14(m, 25.0H, CH 2 CHCH 3 O),4.37-4.00(m,48.2H,OCH 2 CHCH 3 ),2.67-2.55(m,5.8H,C=OCH 2 CH 2 C=O),1.40-1.08(m,72.0,CHCH 3 )). Attached here is DP 20, 10mol% succinic anhydride copolymer 1 H NMR spectroscopy, such as Figure 15 shown. The degree of polymerization was confirmed by NMR, and the number average molecular wei...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| elastic modulus | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com