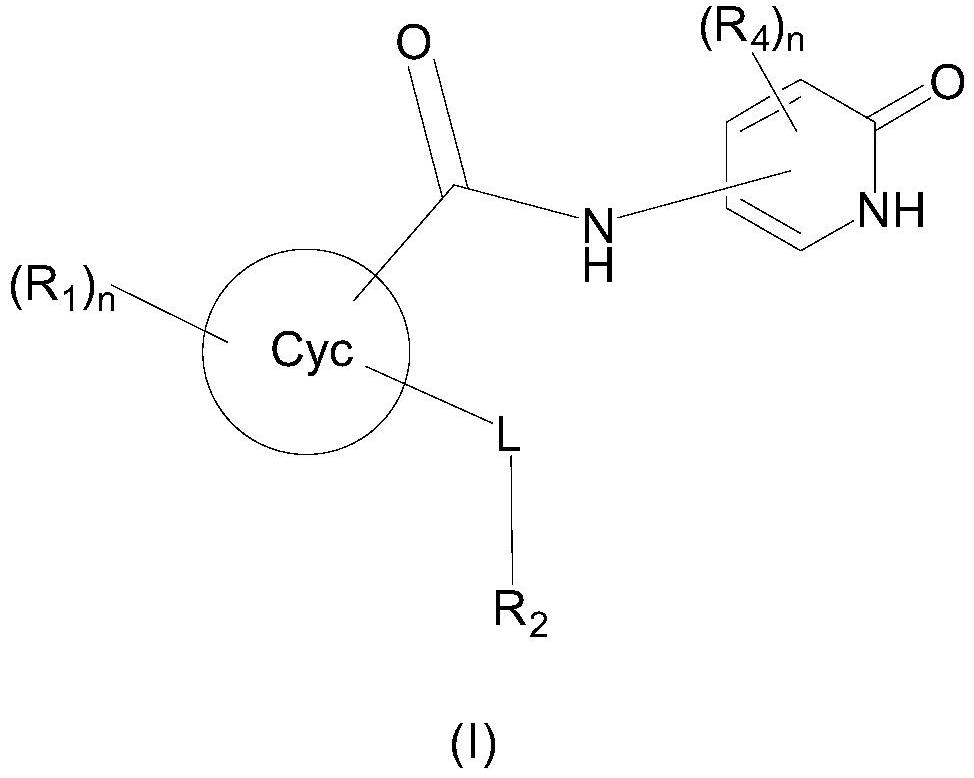
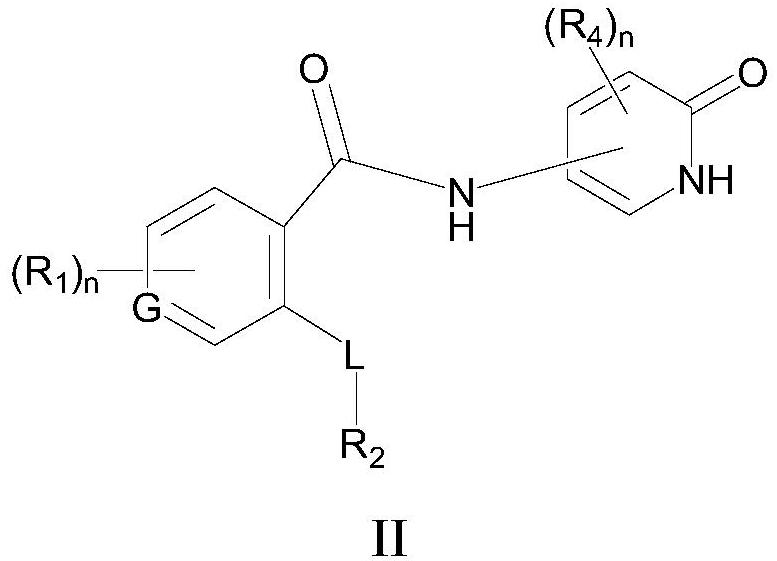
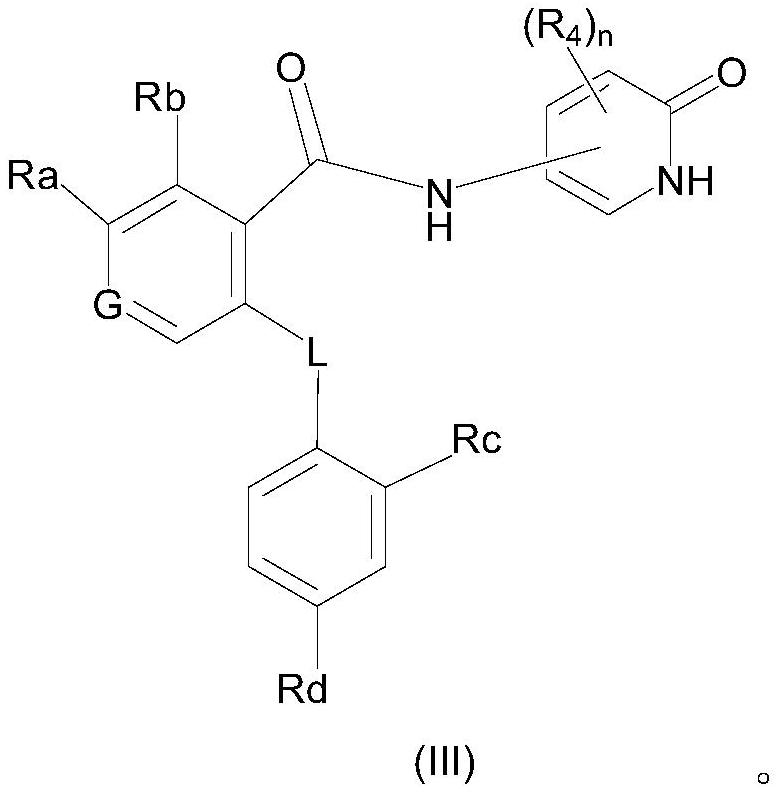
Selective sodium channel regulator, preparation and application thereof
A technology selected from, solvates, applied in the field of selective sodium channel modulators and their preparation and application, capable of solving problems such as poor therapeutic window and lack of isotype selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084]
[0085]
[0086] first step
[0087] Under nitrogen protection, compound 1a (10.00g, 38.90mmol) and potassium carbonate (16.10g, 116.72mmol) were dissolved in N,N-dimethylformamide (100mL), then iodomethane (8.30g, 58.35mmol) was added ), reacted at room temperature for 2 hours. After the reaction, add water (50mL) to quench the reaction, and add ethyl acetate (50mL), separate the layers, extract the aqueous phase with ethyl acetate (30mLx 3), combine the organic phases, dry over anhydrous sodium sulfate, filter, Concentration under reduced pressure gave 1b (12.00 g), yield: 99%.
[0088] 1 H NMR (400MHz, DMSO-d 6 )δ7.66(dd, J=8.7,0.4Hz,1H),7.10(d,J=2.6Hz,1H),6.91–6.84(m,1H),3.85(s,3H).
[0089] second step
[0090] To a solution of compound 1b (12.00 g, 44.28 mmol) in tetrahydrofuran (100 mL) was slowly added dropwise a solution of n-butyllithium tetrahydrofuran (2.5M, 20 mL, 0.50 mmol) under nitrogen protection at -78°C. Stirring was continued for 30 minu...
Embodiment 2
[0109]
[0110] first step
[0111] Compound 2a (200mg, 1.56mmol) was dissolved in toluene (5mL), potassium hydroxide (263mg, 4.68mmol) and benzyl alcohol (336mg, 3.12mmol) were added to the reaction system, and then nitrogen was replaced three times, and the temperature was raised to 120 °C overnight. After the system was cooled to room temperature, water (50 mL) was added to the reaction system and extracted with ethyl acetate (50 mL x 3). After the organic phases were combined, they were washed with saturated brine (50 mL x 1), dried over anhydrous sodium sulfate, and filtered. Concentration under reduced pressure gave a residue, and the residue was purified by column chromatography (ethyl acetate:petroleum ether=0-100%) to obtain compound 2b (212 mg), yield: 58%.
[0112] MS-ESI calculated value [M+1] + 201, measured value: 201.
[0113] second step
[0114] Dissolve 1h (600mg, 1.45mmol) in N,N-dimethylformamide (10mL), then add 2b (300mg, 1.45mmol), 2-(7-benzotriaz...
Embodiment 3
[0121]
[0122] first step
[0123] Dissolve 1f (300mg, 1.17mmol), 3a (148mg, 1.17mmol) in N,N-dimethylformamide (10mL), slowly add cesium carbonate (767mg, 2.35mmol) at 0°C, and replace nitrogen with 0 Stir at room temperature for 2 hours at ℃, dilute the reaction system with water (30mL), and extract with ethyl acetate (50mL x 3), combine the organic phases, wash with saturated brine (20mL x 1), dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure A residue was obtained, and the residue was purified by column chromatography (ethyl acetate:petroleum ether=0-100%) to obtain compound 3b (380 mg), yield: 90%.
[0124] 1 H NMR (400MHz, CDCl 3 )δ7.85(s,1H),7.04–6.90(m,3H),4.48–4.41(m,2H),2.20(s,3H),1.42–1.36(m,3H).
[0125] second step
[0126] 3b (380 mg, 1.05 mmol) was dissolved in tetrahydrofuran (5 mL), 1N aqueous sodium hydroxide solution (5 mL) was added, nitrogen was replaced and stirred at room temperature for 2 hours. Add 6N dilute hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com