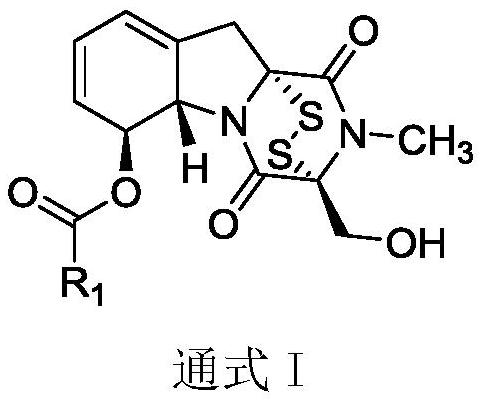
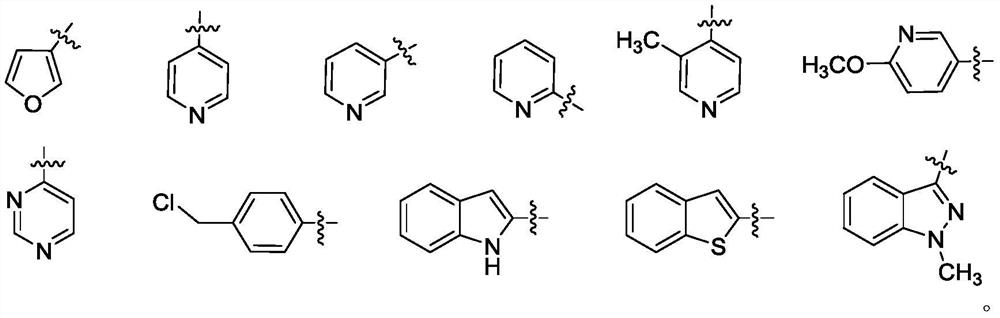
Gliotoxin 6-aromatic ring carboxylic ester series derivatives and preparation method thereof
A technology for aromatic ring carboxylic acid and gliotoxin, which is applied to the derivatives of epipolythiodiketopiperazine natural products gliotoxin, gliotoxin 6-aromatic carboxylic acid ester compounds and the field of preparation thereof , which can solve the problems of poor stability, large toxic and side effects, weak research on synthesis and structure-activity relationship, etc., and achieve the effect of expanding the structure type and good application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
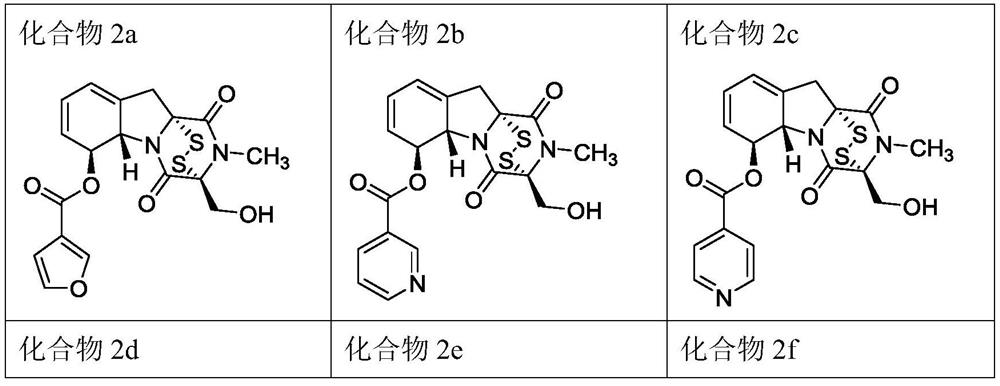
[0024] 150 mg of gliotoxin was weighed and dissolved in 3 mL of dichloromethane, stirred and dissolved at room temperature, and 1.1 eq of 3-furancarboxylic acid, 1 eq of DCC and 0.1 eq of DMAP were added. During the reaction, TLC monitoring was performed every 30 min, and the reaction was completed in 1-2 h. 30 mL of dichloromethane was added to the reaction system to dilute, and the reaction system was diluted with saturated NH 4 The Cl solution was washed three times, and the aqueous layer was back-extracted once with dichloromethane. All organic phases were combined and washed three times with saturated NaCl solution, and the organic phases were combined. Dry with anhydrous magnesium sulfate for 12h, concentrate the organic phase and add 2 times silica gel, and use column chromatography to separate and purify light yellow compound 2a, yield 58%, m.p.: 105.2-106.1°C; IR (KBr)ν max :3425,2921,1735,1685,1378,1302,1160,1077,873cm -1 . 1 H NMR (400MHz, DMSO-d 6 )...
Embodiment 2
[0026]
[0027] Substitute 3-picolinic acid for 3-furancarboxylic acid, and other operations are the same as in Example 1, to obtain light yellow solid 2b with a yield of 57%. m.p.: 102.0-103.1°C; IR(KBr)ν max :3726,3703,3420,2920,1734,1685,1590,1429,1380,1274,1192,1111,1024,720,668cm -1 . 1 H NMR (400MHz, DMSO-d 6 )δ(ppm): 9.17(d, J=2.2Hz, 1H), 8.92(dd, J=4.8, 1.7Hz, 1H), 8.38(dt, J=8.0, 2.0Hz, 1H), 7.67(dd, J=8.0, 4.8Hz, 1H), 6.09 (dt, J=5.7, 3.0Hz, 1H), 6.01 (ddd, J=9.8, 5.0, 2.8Hz, 1H), 5.69 (d, J=9.7Hz, 1H) ),5.54(s,1H),5.38(d,J=12.8Hz,1H),5.22(d,J=12.8Hz,1H),4.93(d,J=13.1Hz,1H),4.61(d,J =13.3Hz,1H),3.72(ddq,J=17.6,3.4,1.8Hz,1H),3.24(d,J=4.4Hz,3H),2.55(p,J=1.9Hz,1H). 13 C NMR (101MHz, DMSO-d 6 )δ(ppm): 165.08, 163.55, 163.17, 154.19, 150.12, 137.13, 132.40, 129.55, 124.71, 124.10, 123.59, 118.97, 75.88, 75.74, 72.64, 69.46, 60.65, 35.78 ESI-HRMS:m z cacld.For C 19 H 17 N 3 O 5 S 2 [M+H] + :432.0682,found432.0689.
Embodiment 3
[0029]
[0030]Substitute 4-picolinic acid for 3-furancarboxylic acid, and other operations are the same as in Example 1, to obtain pale yellow solid 2c, yield 62%, m.p.: 168.1-169.2°C; IR(KBr)ν max :3726,3417,2927,2850,1735,1704,1685,1672,1626,1574,1405,1379,1354,1324,1271,1190,1121,1094,1061,708cm -1 . 1 H NMR (400MHz, DMSO-d 6 )δ(ppm): 8.93–8.86 (m, 2H), 7.94–7.88 (m, 2H), 6.08 (dt, J=5.8, 3.0Hz, 1H), 6.00 (ddd, J=8.1, 4.9, 2.8Hz ,1H),5.68(d,J=9.7Hz,1H),5.38(d,J=12.8Hz,1H),5.21(d,J=12.8Hz,1H),4.92(d,J=13.2Hz,1H) ), 4.60(d, J=13.2Hz, 1H), 3.77-3.68(m, 1H), 3.38(s, 2H), 3.23(s, 3H). 13 CNMR (101MHz, DMSO-d 6 )δ(ppm): 163.15, 150.94, 132.38, 129.55, 123.59, 122.58, 118.98, 75.87, 75.65, 72.64, 69.48, 61.00, 35.78, 28.16.ESI-HRMS: m / zcacld.For C 19 H 17 N 3 O 5 S 2 [M+H] + : 432.0682, found 432.0687.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com