Protein expression method
A technology of antibody and supplementary culture medium, applied in the biological field, can solve the problems affecting the therapeutic effect of protein drugs and the side effects of protein drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the screening of culture medium kind
[0038] Purchase CHOM-B series products and CHOM-S series products of serum-free, animal protein-free, animal-derived component-free medium from Shanghai Maitai Junao Biotechnology Co., Ltd., select CHOM-B01, CHOM-B02, CHOM-B03 and CHOM-B01 S01, CHOM-S03, CHOM-S04 products.
[0039] According to the test results of using CHOM-B series medium and CHOM-S series medium in the early stage, the basic medium and supplementary medium were further screened. According to the type of basal medium and supplementary culture and the ratio of each component, the basal medium is divided into CHOM-B01:CHOM-B02 as 1:1, 1:3, 1:5 and CHOM-B01:CHOM-B03 as 1:0.1, 1:0.5, 1:1, divide the supplementary medium into CHOM-S01:CHOM-S03 as 1:0.3, 1:0.6, 1:1 and CHOM-S01:CHOM-S04 as 1:1, 1:4, 1:7.
[0040] CHO cells were cultured using the above-mentioned different proportions of medium, and after basic culture and supplementary culture, the cel...
Embodiment 2
[0047] Embodiment 2, protein expression method
[0048] Select the basal medium and the supplementary medium CHOM-B01:CHOM-B02 after the screening of Example 1 to be 1:3, CHOM-B01:CHOM-B03 to be 1:1, and the supplementary medium CHOM-S01:CHOM-S03 to be 1:0.3, CHOM-S01:CHOM-S04 is 1:4, large-scale production and expression of the target protein.
[0049] The large-scale production and expression of proteins mainly includes the stages of cell expansion and perfusion culture, and the stage of supplementary culture.
[0050] 1. Cell expansion:
[0051] 1) Recovery of working cells: Take out 1 working cell from the working cell bank, put it in a 37°C water bath, and melt it; use 5ml of recovery culture medium, centrifuge to change the medium, inoculate into a 100ml~150ml shake flask, add 10~ 15ml; the shaker flask is placed in a carbon dioxide shaker for shaking culture, the temperature is 37°C, 70~170rpm, and the carbon dioxide concentration is 2~10%.
[0052] 2) First-class se...
Embodiment 3
[0061] Embodiment 3, the expression of Pan-P antibody
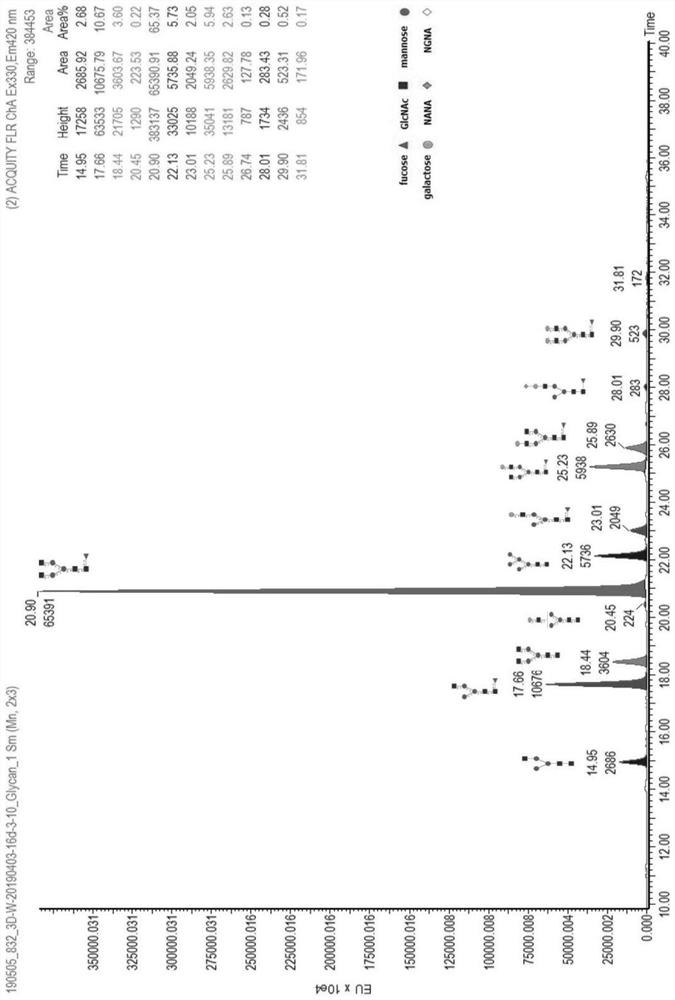
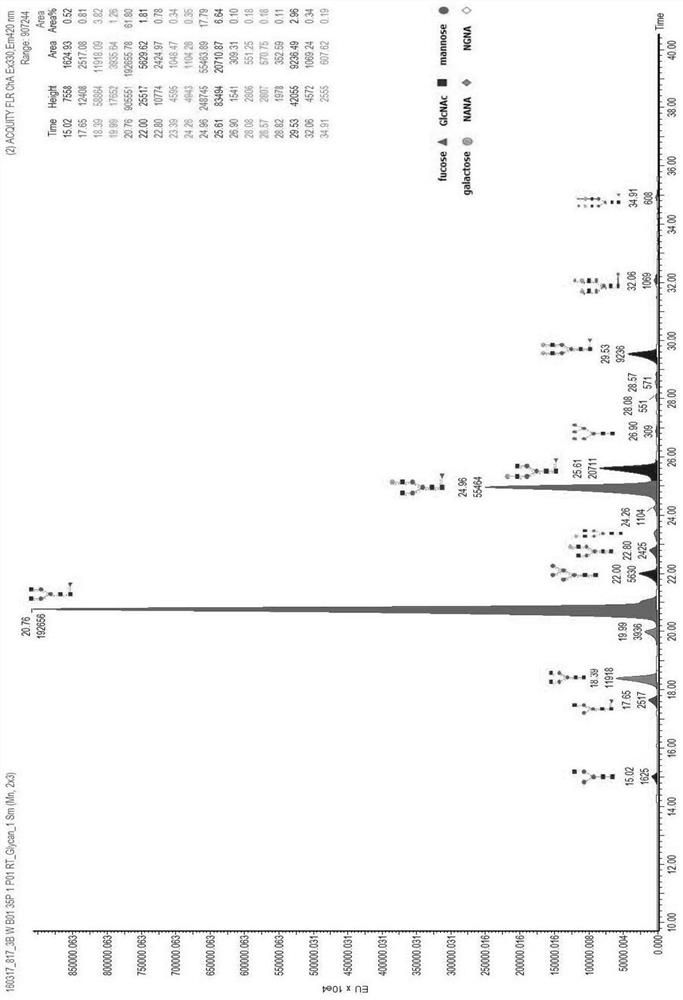
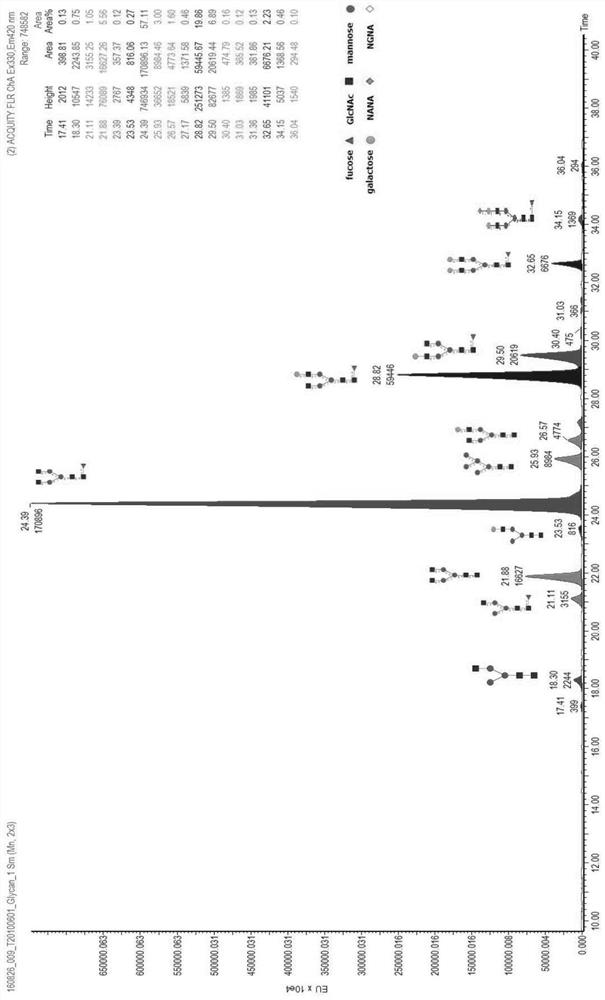
[0062] Taking the Pan-P antibody as an example, use the above-mentioned different basal medium and supplementary medium to culture and express it in CHO cells, and detect the glycoform of the Pan-P antibody obtained by culturing and expressing it.
[0063] Pan-P is the fully human epidermal growth factor receptor EGFR monoclonal antibody disclosed in the patent application number CN201410724477.4, and the expression vector containing the Pan-P gene sequence was obtained according to the method disclosed in the patent CN201410724477.4, and transfected into CHO cells for expression.
[0064]For large-scale production and expression of Pan-P antibody, CHO host cells containing Pan-P antibody gene sequence are cultivated and expressed by basal culture and supplementary culture. The basal medium is based on CHOM-B01:CHOM-B02 at 1:3 or CHOM-B01:CHOM-B03 at 1:1; the supplementary medium is: CHOM-S01:CHOM-S03 at 1:0.3 or CHOM-S0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com