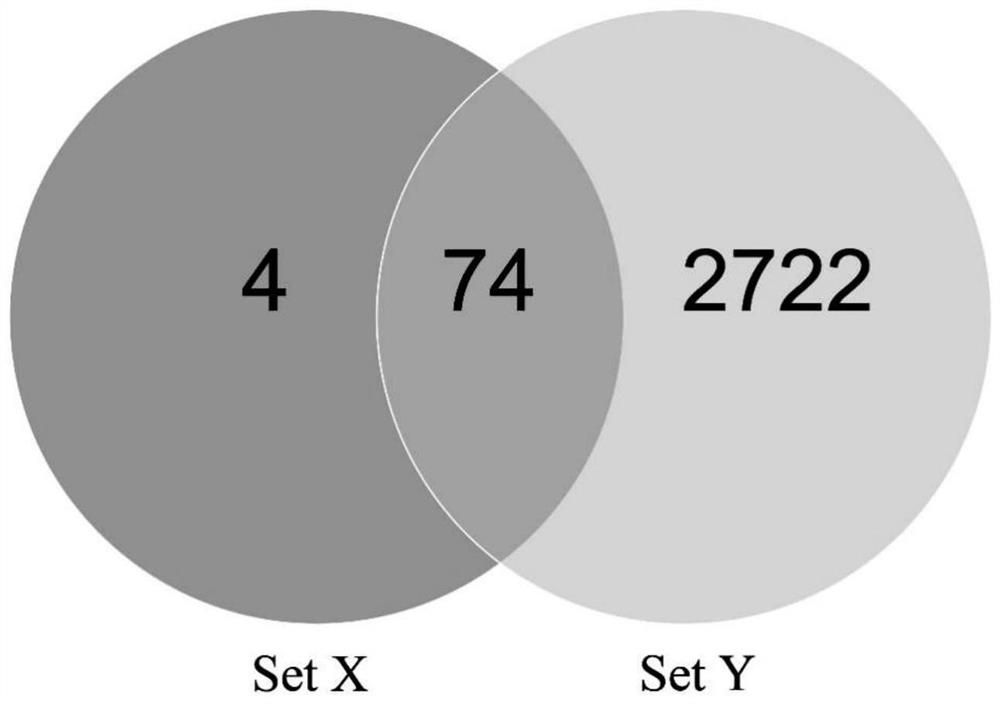
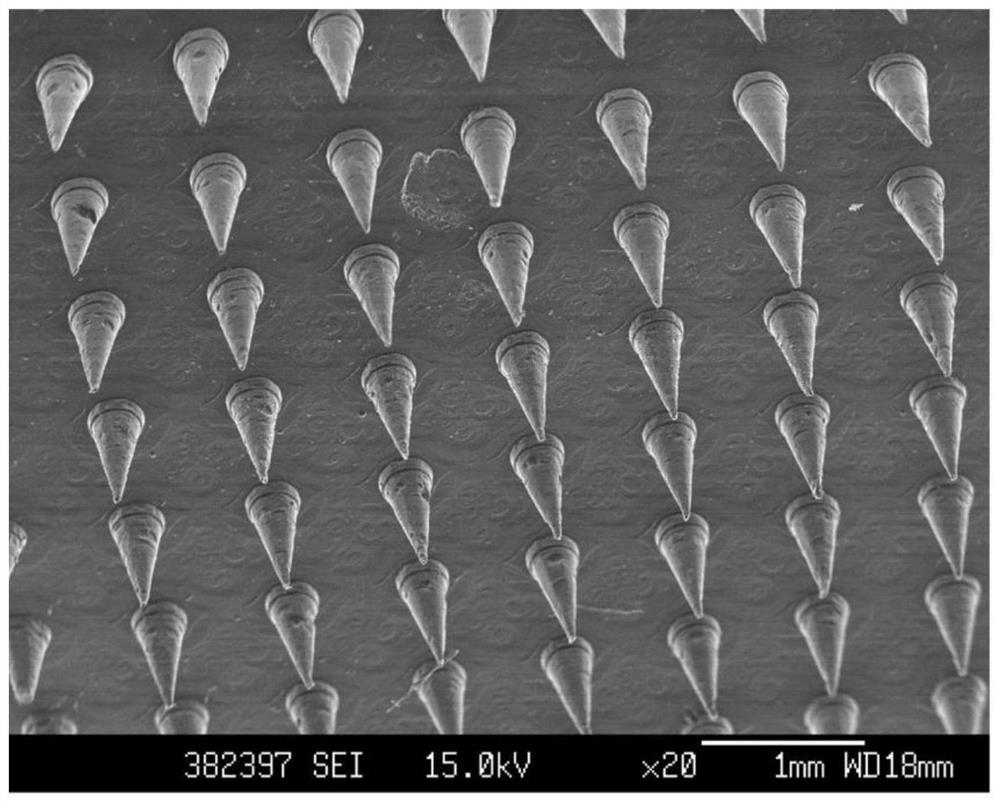
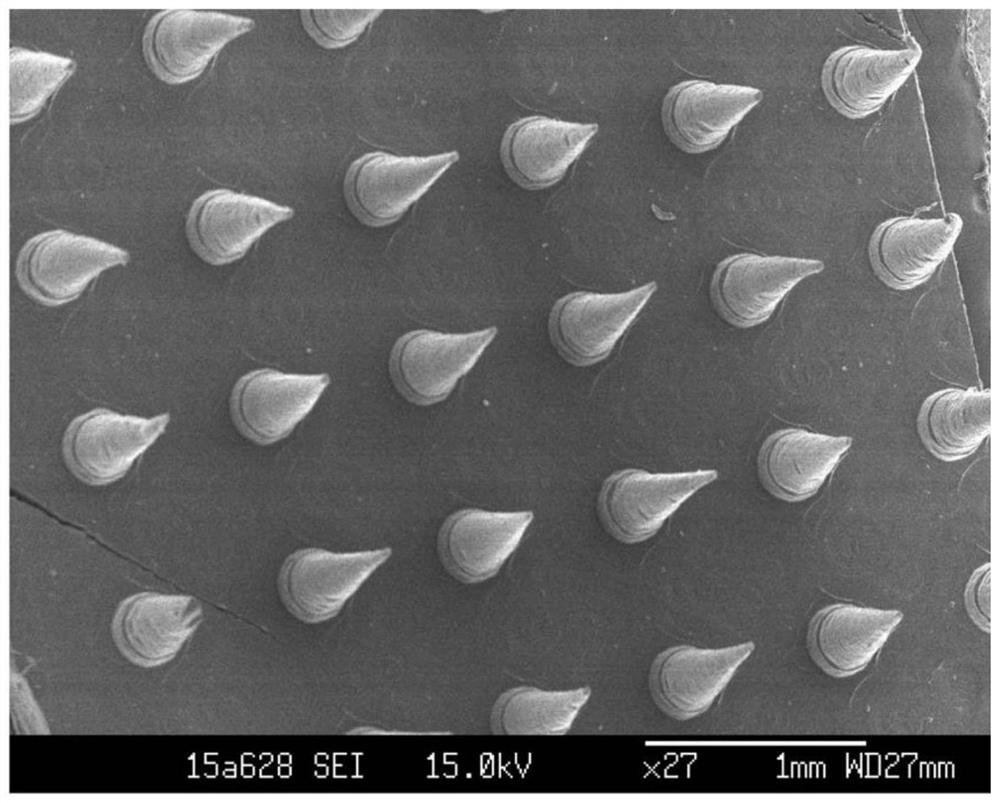
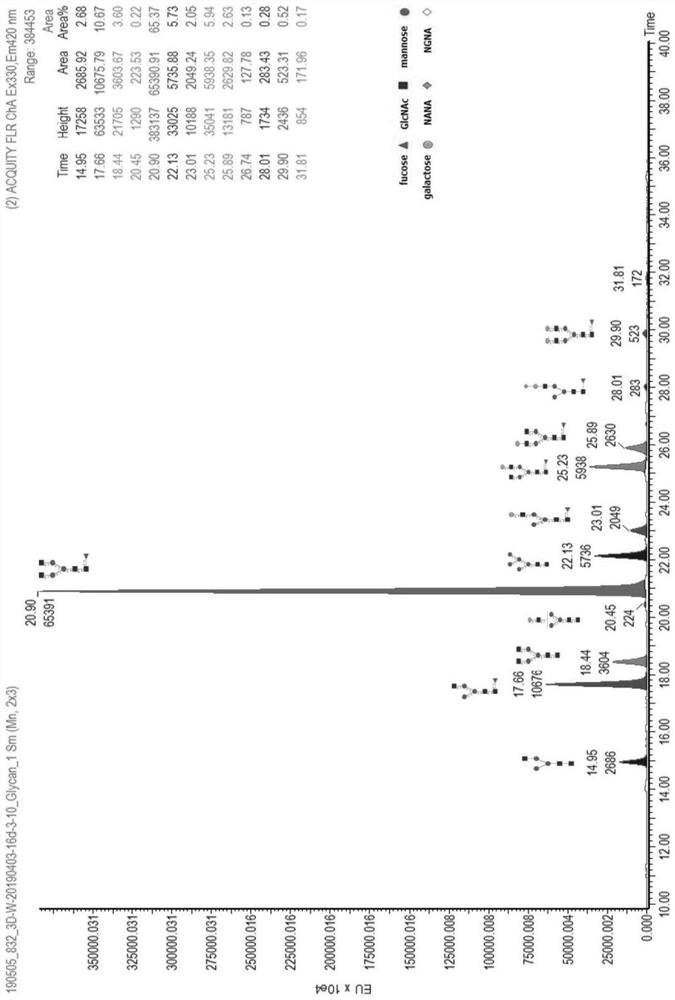
Patents
Literature
38 results about "Apoferritins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
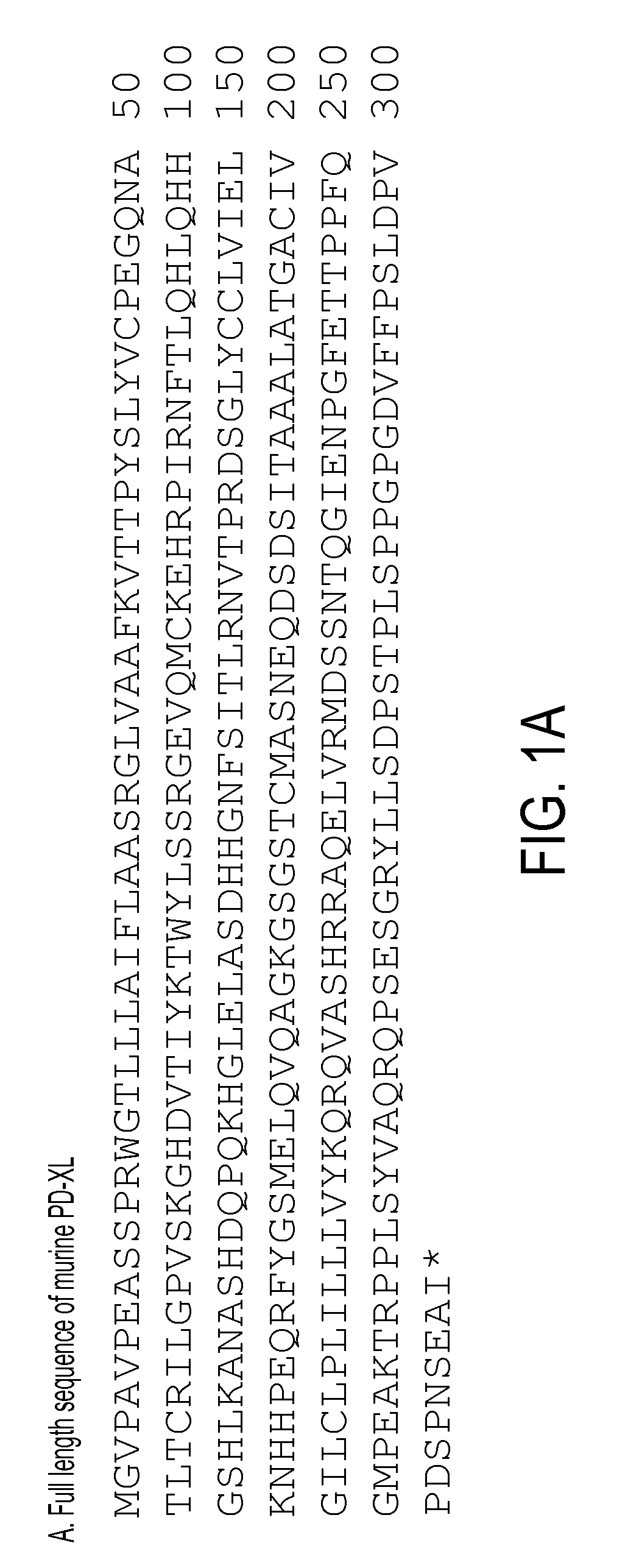
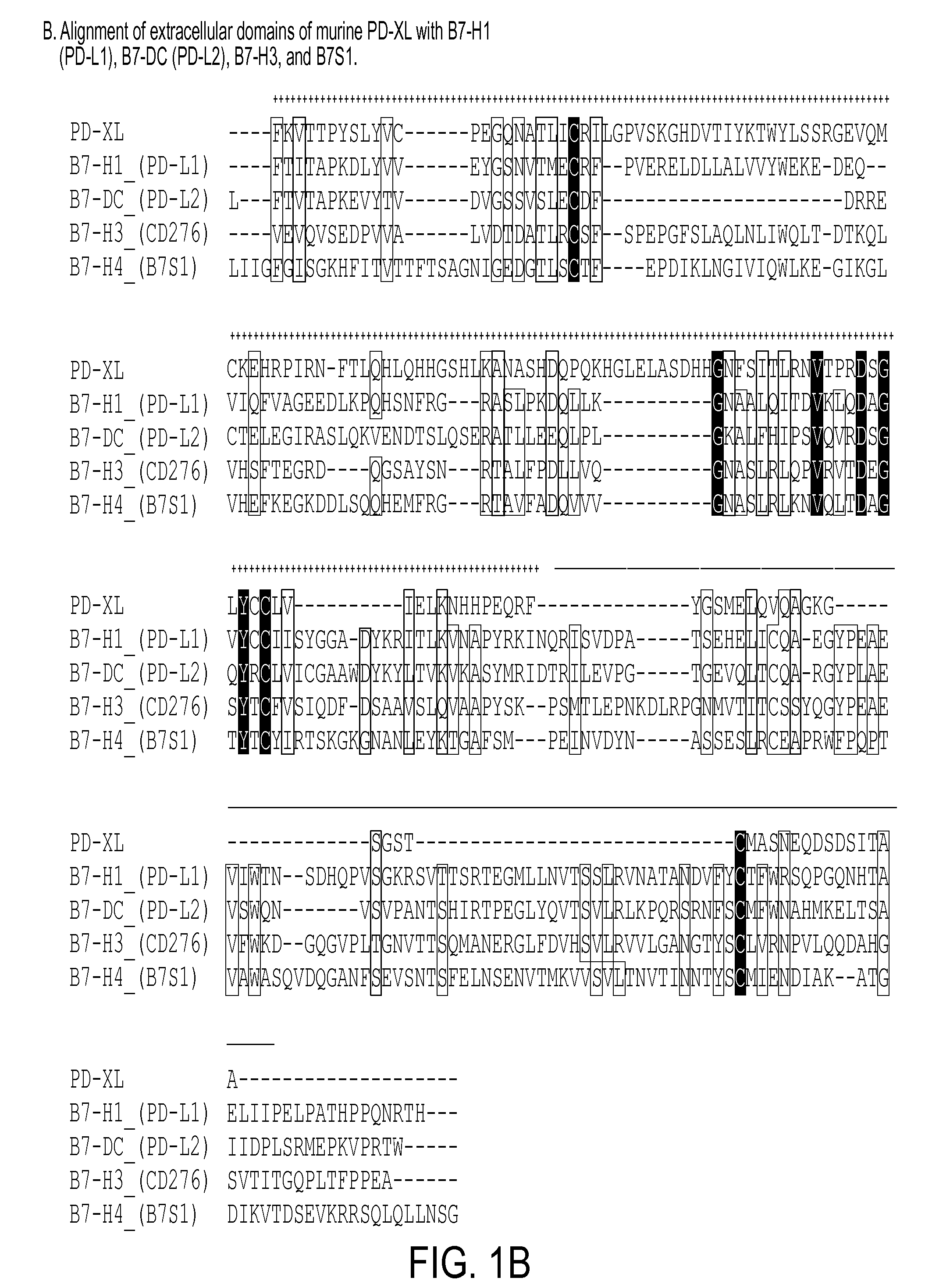
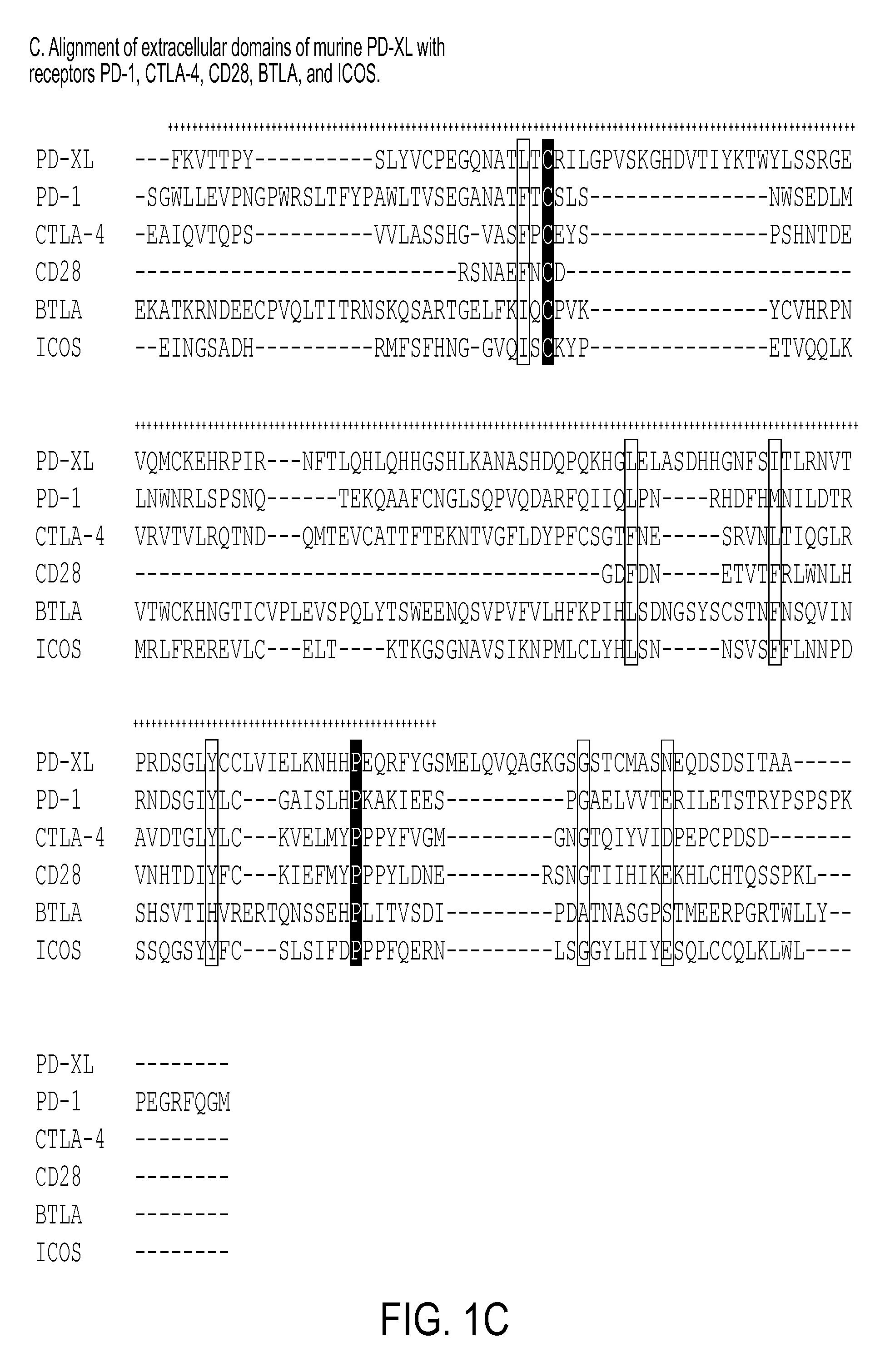
Regulatory t cell mediator proteins and uses thereof
ActiveUS20110027278A1Eliminate the effects ofPeptide/protein ingredientsAntibody mimetics/scaffoldsRegulatory T cellAllergy
The present invention relates to novel regulatory T cell proteins. One protein, designated PD-L3, resembles members of the PD-L1 family, and co-stimulates αCD3 proliferation of T cells in vitro. A second, TNF-like, protein has also been identified as being upregulated upon αCD3 / αGITR stimulation. This protein has been designated Treg-sTNF. Proteins, antibodies, activated T cells and methods for using the same are disclosed.In particular methods of using these proteins and compounds, preferably antibodies, which bind or modulate (agonize or antagonize) the activity of these proteins, as immune modulators and for the treatment of cancer, autoimmune disease, allergy, infection and inflammatory conditions, e.g. multiple sclerosis is disclosed
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Method for identifying specific peptides of Chinese medicine containing protein
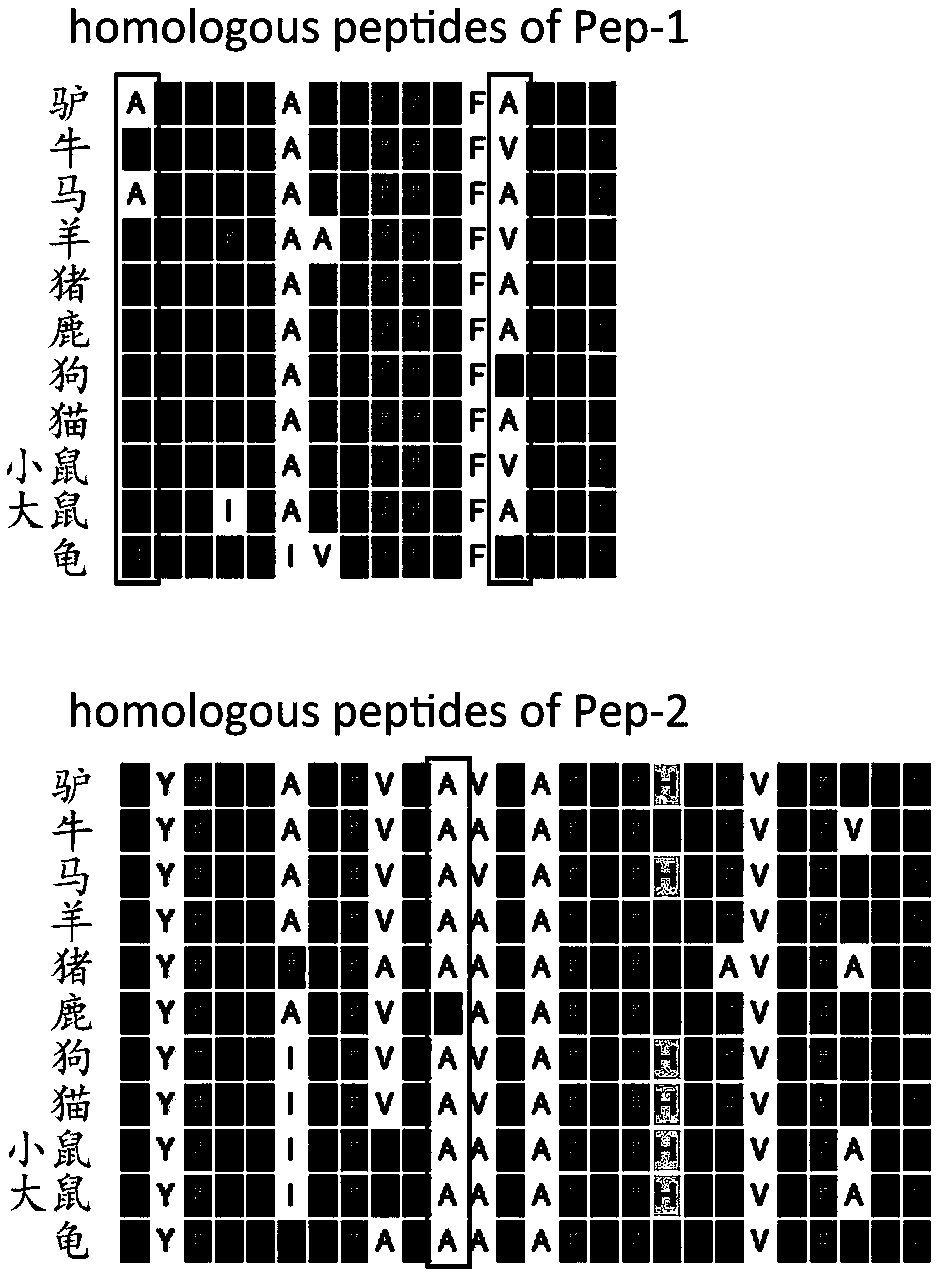
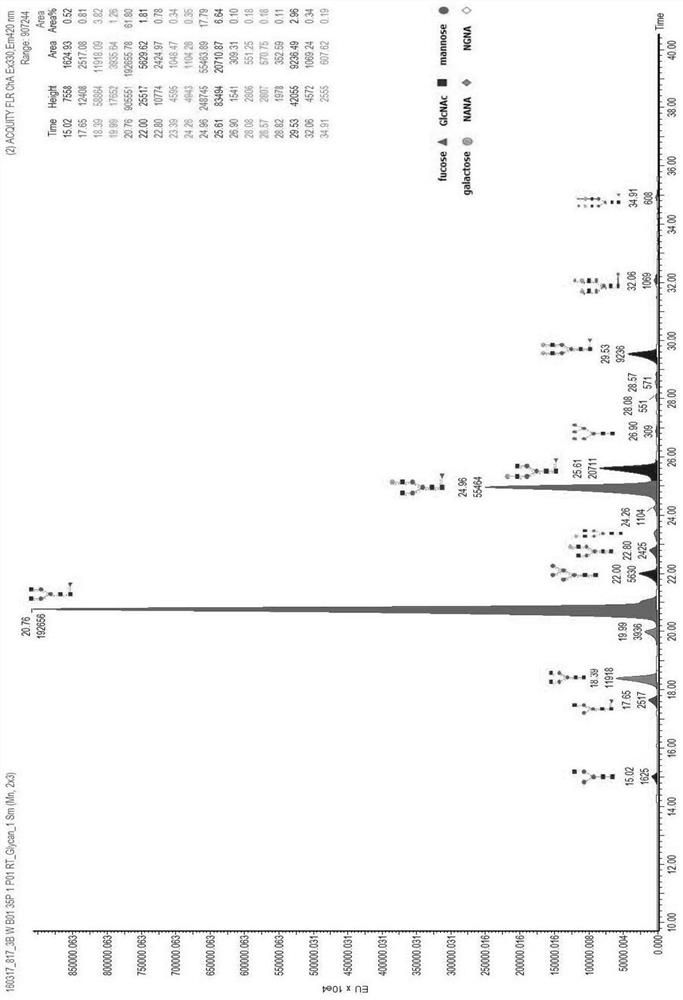
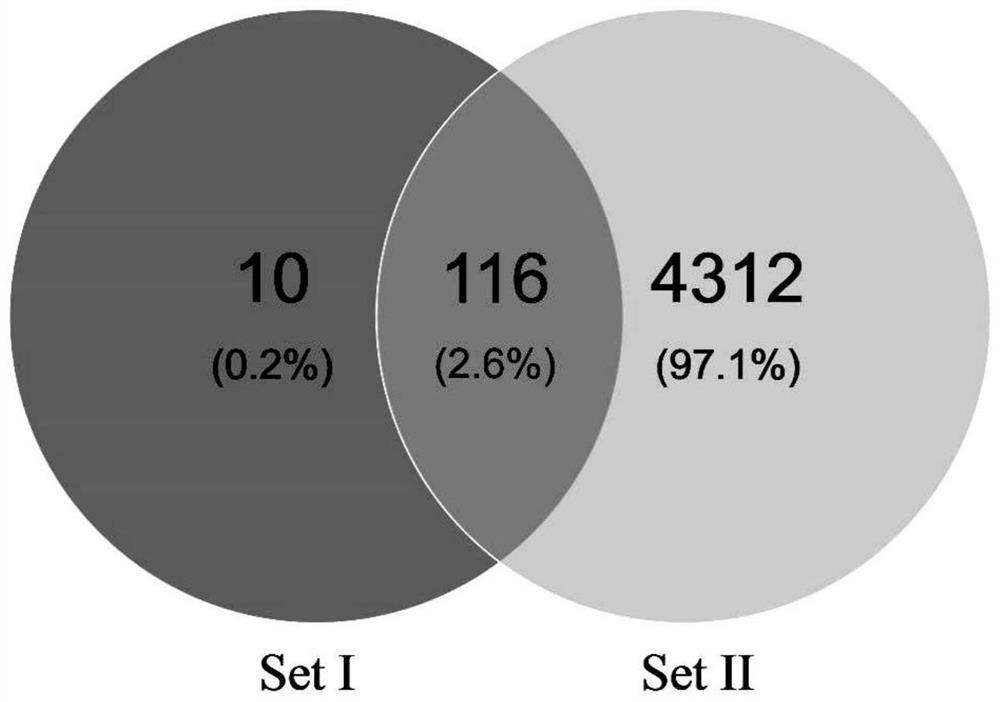
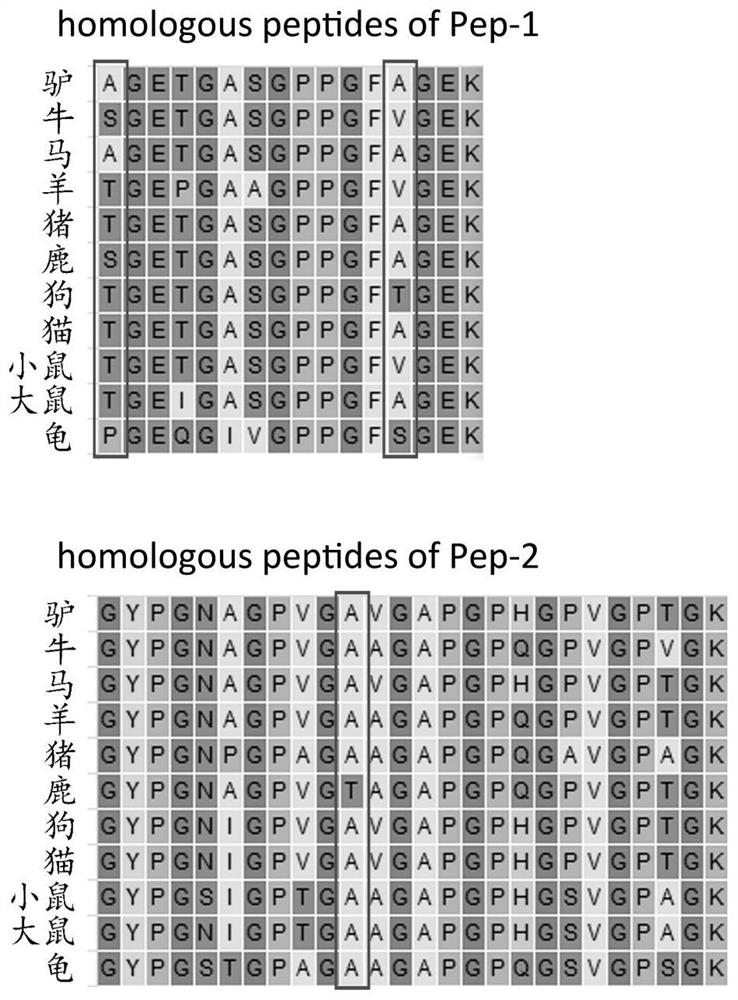
The invention discloses a method for identifying specific peptides of a Chinese medicine containing protein. The method comprises the following steps of: screening through a large number of experiments; taking a sample and performing enzymatic hydrolysis by using preferred enzyme; performing a desalination treatment; performing a high-throughput identification on peptide sequences using the best Nano LC-MS / MS method; verifying specific peptides by comparing differences in homologous protein sequences between different species of different samples; validating the specificity of the specific peptides in authentic products using the preferred LC-QQQ MS analysis. The method is scientific and easy to operate, and can be used to identify authentic and fake products of Chinese medicine, animal drugs, foods and aquatic products, and has important application value.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Protein-medicament-carrying PLGA composite microspheres and preparation method thereof
InactiveCN102727899AThe preparation process is stableThe preparation process is feasiblePowder deliveryPeptide/protein ingredientsProlonged-Action PreparationsMicrosphere
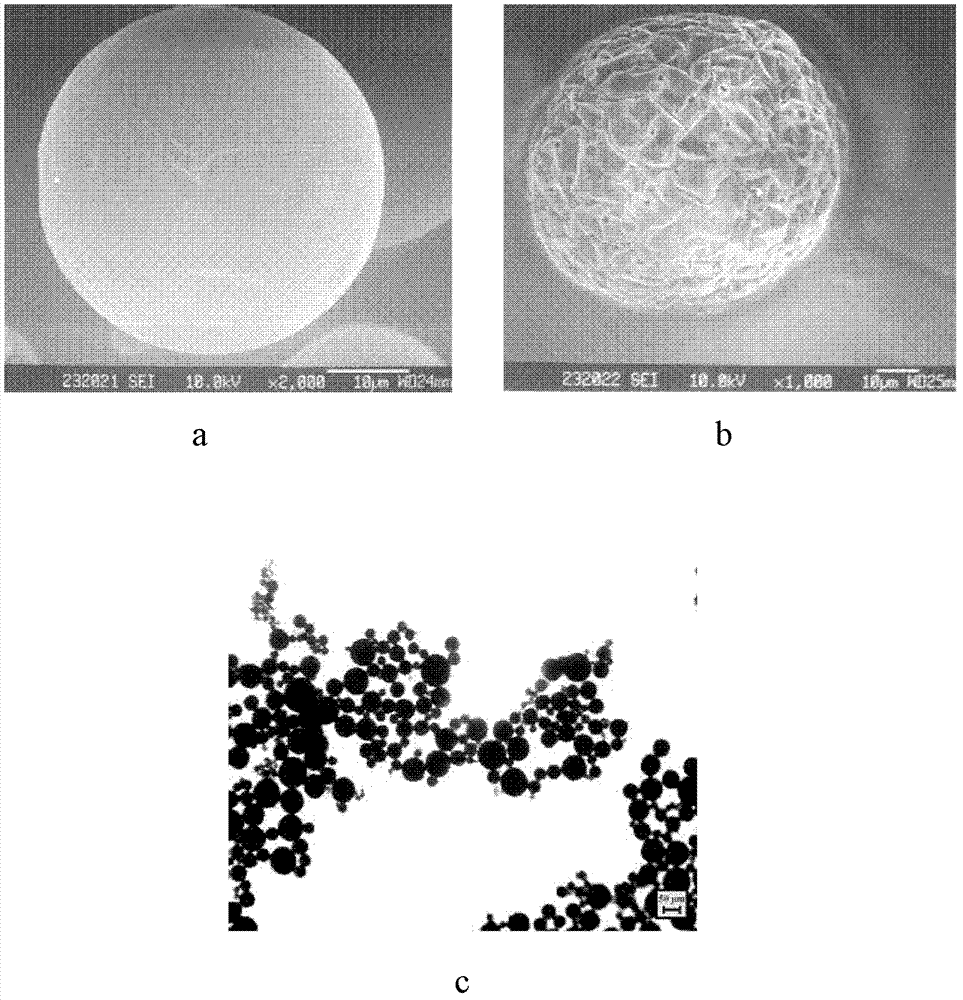
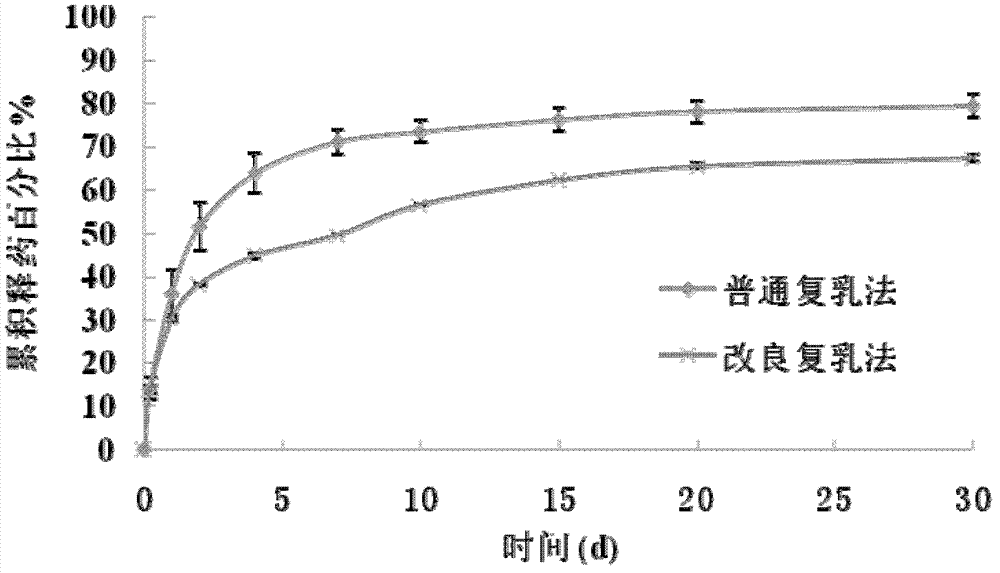
The invention discloses protein-medicament-carrying PLGA composite microspheres and a preparation method thereof. According to the invention, an improved multiple emulsion-solvent evaporation method is adopted. On the basis of a multiple emulsion method, a principle that sodium alginate and calcium ions are subjected to chelating and form sustained-release gel is adopted; PLGA is adopted as a microsphere carrier; lyophilized injection-use recombinant human interferon-alpha, bovine serum albumin and the like are adopted as encapsulation objects; and the medicament-carrying microspheres are prepared. The microspheres are advantaged in round appearance, and uniform particle size distribution. An average particle size distribution is 70 micrometers, a medicament-carrying rate is above 0.6%, an encapsulation rate reaches approximately 50%, and an in-vitro medicament releasing performance satisfies the characteristic of prolonged-action preparations.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Immune capture composition, preparation method, kit and application
ActiveCN111381024AGood sealingImprove closureMaterial analysisAgainst vector-borne diseasesMedicineChemical compound
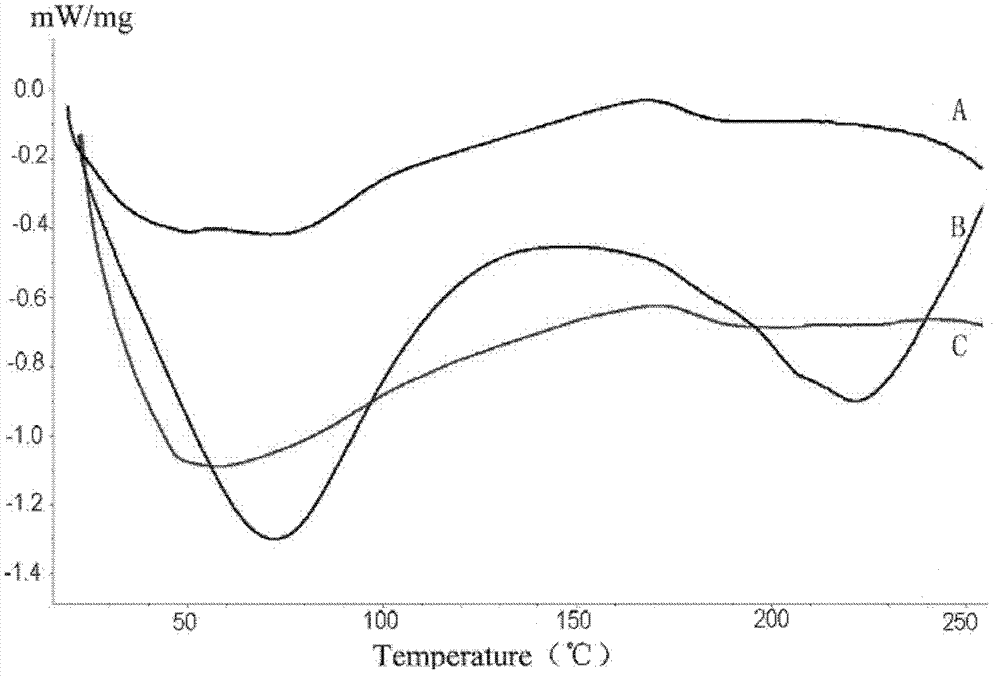
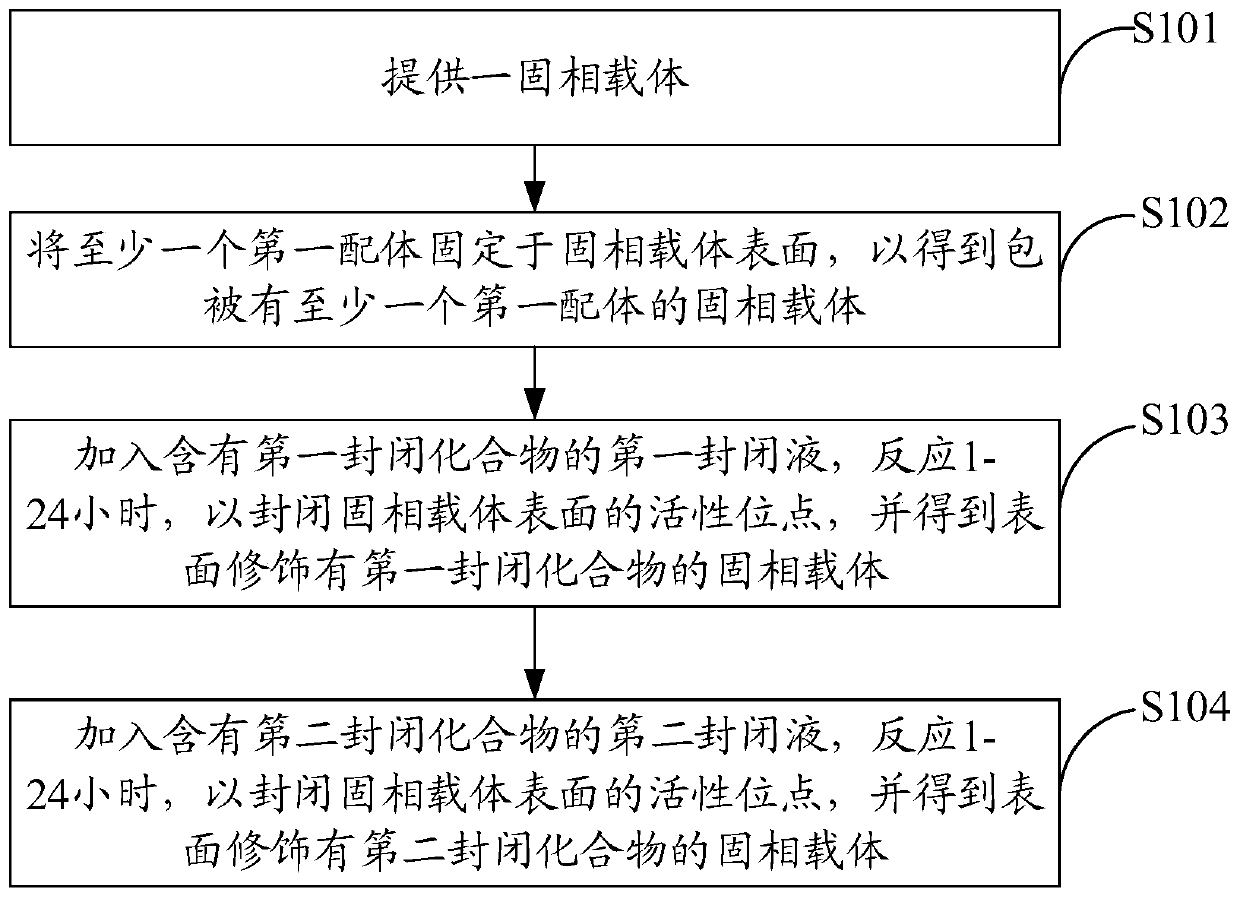
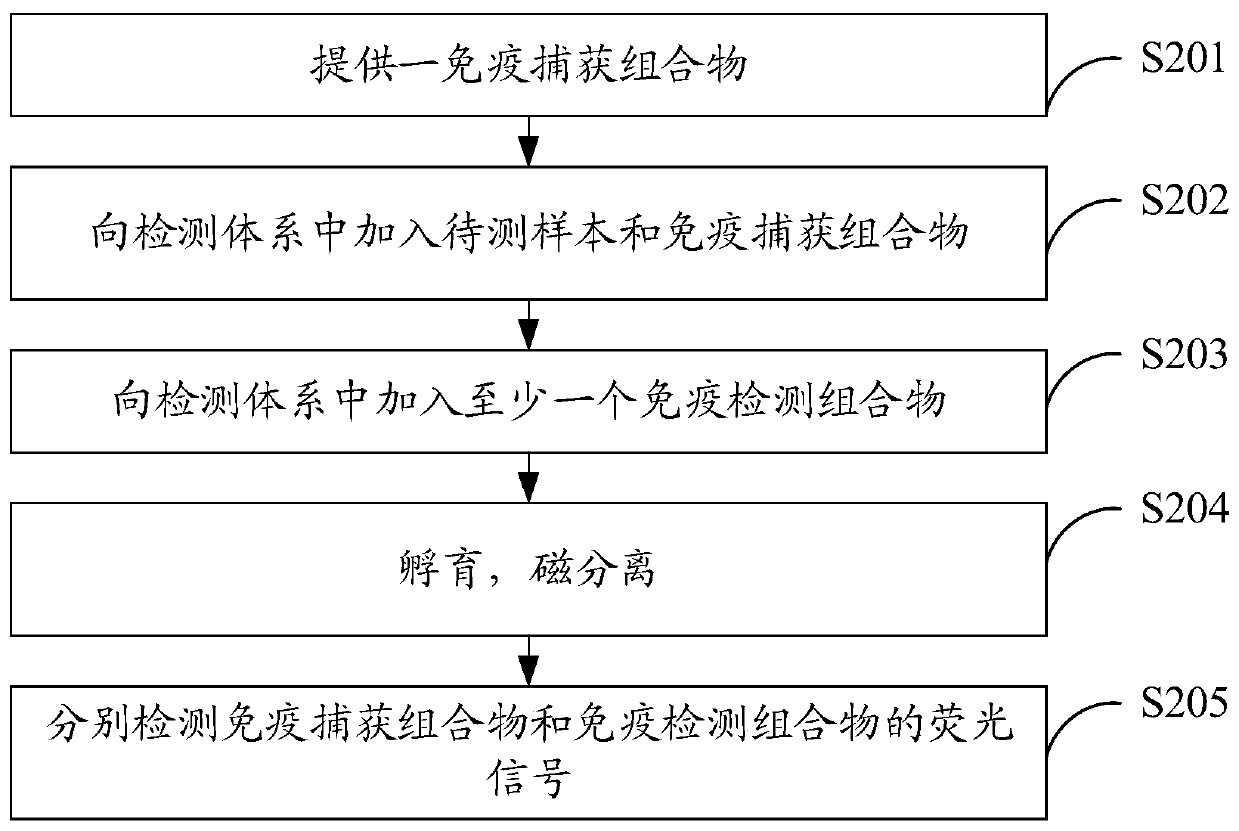
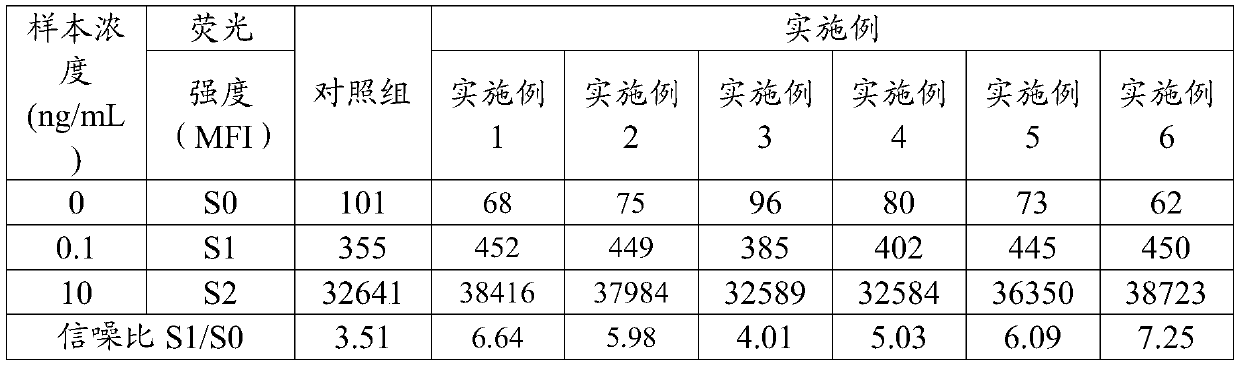
The invention discloses an immunodetection composition, a preparation method, a kit and application. The immune capture composition comprises: a solid-phase carrier; the at least one first ligand thatis fixed on the surface of the solid-phase carrier; the first blocking compound and the second blocking compound that are modified on the surface of the solid-phase carrier and are used for blockingactive sites on the surface of the solid-phase carrier, wherein the first blocking compound is a polyhydroxy saccharide compound or a protein compound, the second blocking compound is a polyhydroxy saccharide compound or a protein compound, and the first blocking compound is different from the second blocking compound. By means of the mode, the sealing effect can be effectively improved, and the accuracy of the detection result is improved.
Owner:SHENZHEN DYMIND BIOTECH
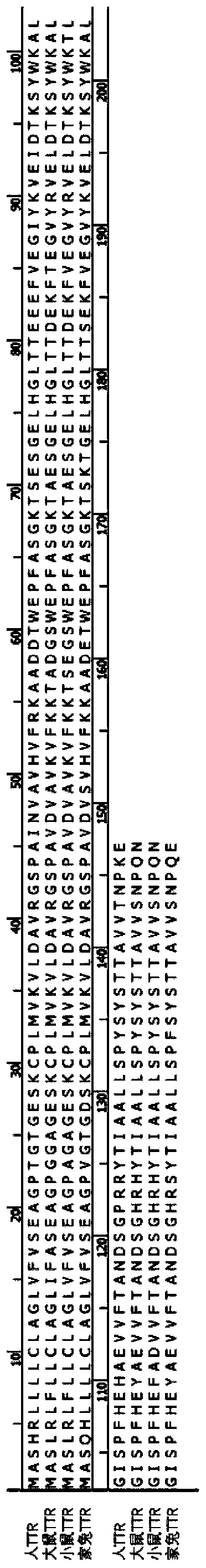
Application of transthyretin in aspects of entering eye and preparing drop
ActiveCN111437398AGood biocompatibilityHigh biosecuritySenses disorderAntibody mimetics/scaffoldsDiseaseAmino acid composition
The invention provides application of transthyretin in the aspect of serving as a carrier for a protein and / or polypeptide drug to enter an eye through an eye barrier. The transthyretin is a protein consisting of amino acid as shown in SEQ ID NO:1 or a mutation thereof or a modification thereof. The invention further provides application of the transthyretin and / or a fusion protein of the transthyretin and a drug to preparation of a drop and a drop. The drug is the protein and / or polypeptide drug. The transthyretin has good biocompatibility and safety in human bodies, can effectively convey aforeign protein and / or polypeptide into the eye and achieves an effect of treating eye diseases. In an eye dropping manner, when the eye diseases such as DR (Diabetic Retinopathy), AMD (Age-related macular degeneration), ROP (Retinopathy of Prematurity) and the like are treated, the transthyretin can enter a vitreous body and eye ground across a cornea barrier so as to obviously inhibit eyeball retina leakage, obviously reduce the number of retinal new blood vessels and effectively relieve the pathological phenomena of the eye diseases.
Owner:易舟(上海)生物医药有限公司
Sphingosine kinase 1 and its fusion protein and application
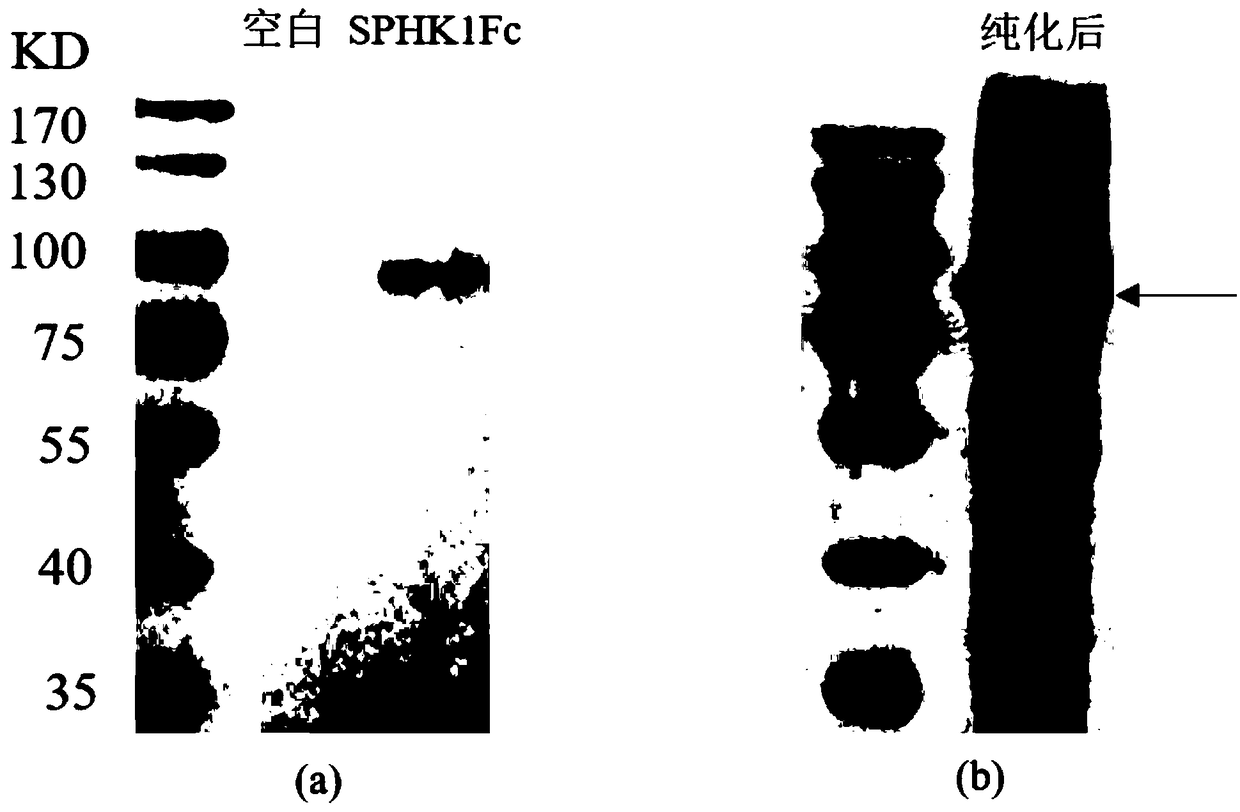
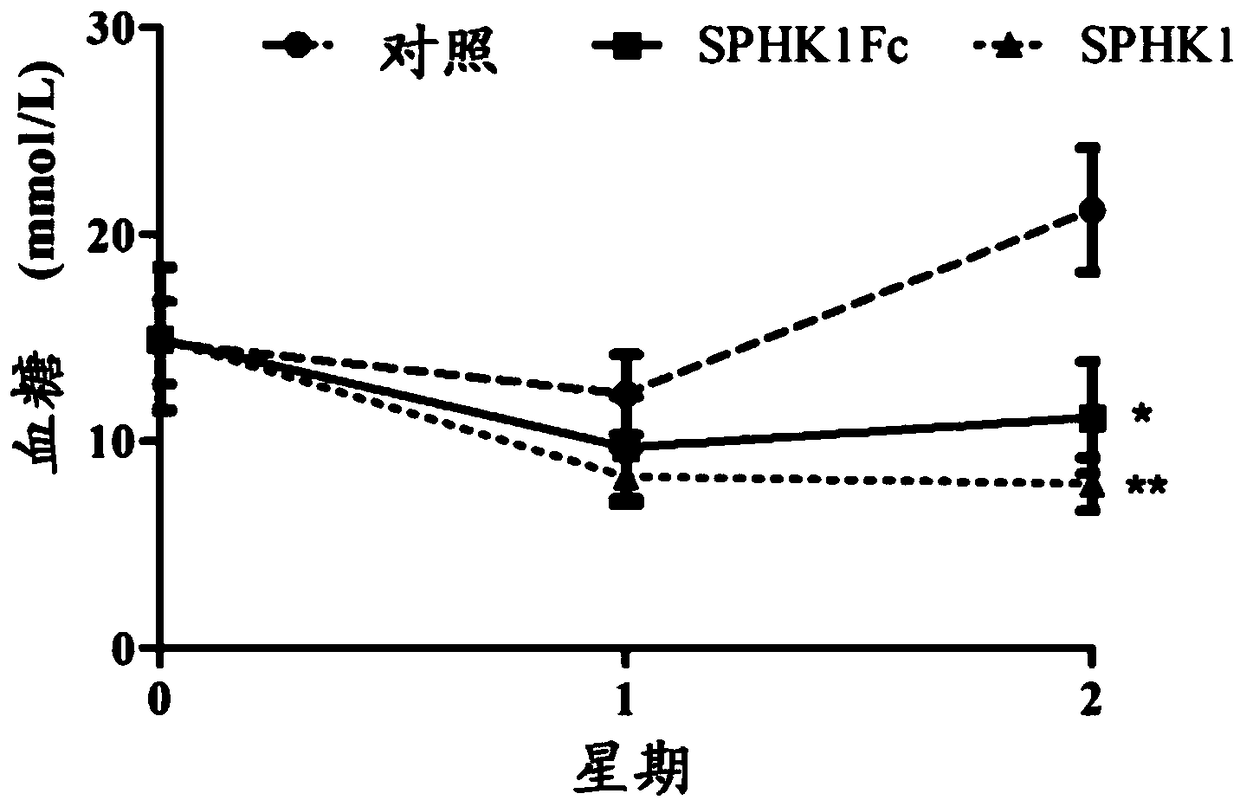
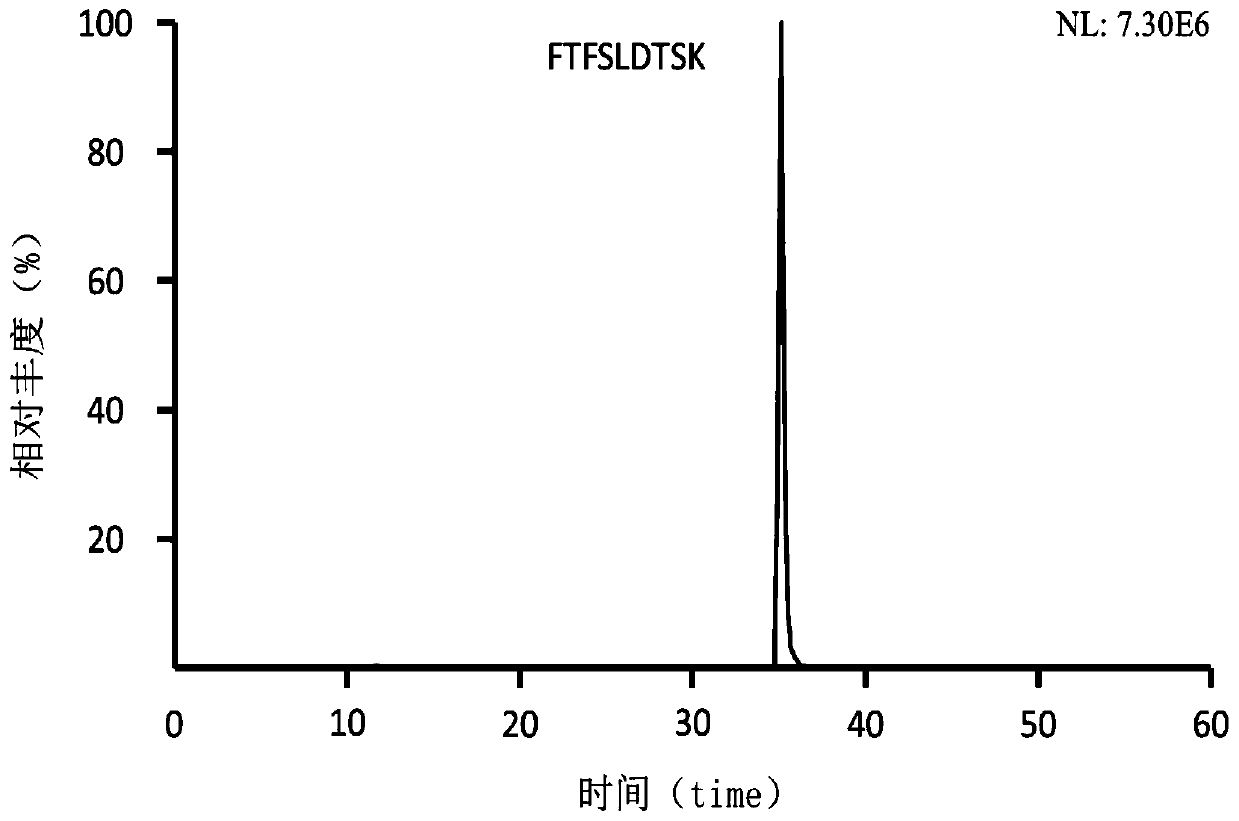
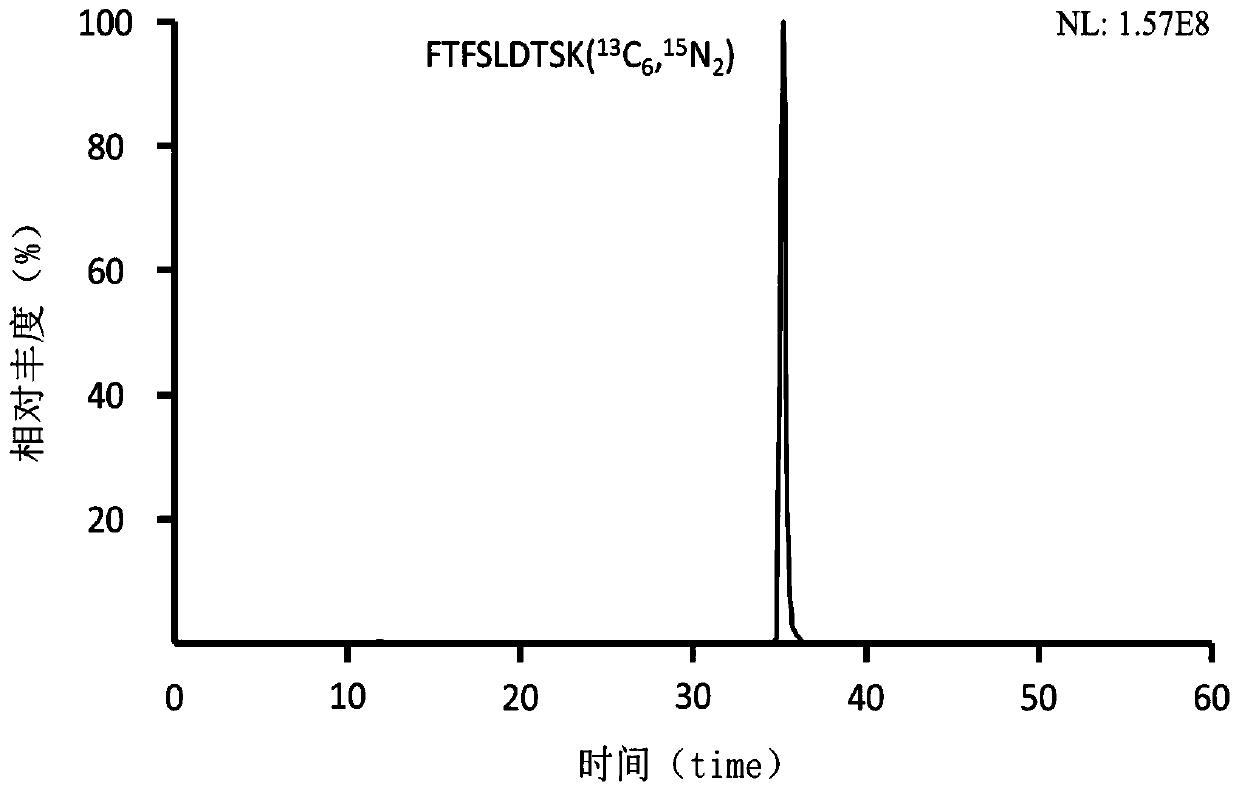
ActiveCN109355269AGood hypoglycemic effectWeight loss effectPolypeptide with localisation/targeting motifPeptide/protein ingredientsPharmaceutical SubstancesBiomedicine
The invention belongs to the technical field of biomedicine and particularly relates to sphingosine kinase 1 and its fusion protein and application, namely sphingosine kinase 1 and application of an active amino acid sequence having its activity in the preparation of protein drugs to prevent and / or treat obesity, hyperlipemia or diabetes mellitus. The invention also provides a protein drug comprising sphingosine kinase 1 or an ammino acid sequence having its activity. The invention also provides application of the protein drug, an encoding gene, an expression construction and a host cell in the preparation of drugs to prevent and / or treat obesity, hyperlipemia or diabetes mellitus. It is discovered herein that sphingosine kinase 1 and its fusion protein have significant glucose-lowering and weight-reducing effects and are applicable to the preparation of protein drugs to control obesity and other metabolic diseases as well as diabetes mellitus.
Owner:北京华奥玄德生物医药科技有限公司
Preclinical pharmacokinetic study mass spectrometry analysis method of bevacizumab
InactiveCN110045028AEase of handlingSimple methodComponent separationLiquid chromatography mass spectroscopyHydrolysis
The invention discloses a pharmacokinetic mass spectrometry analysis method of monoclonal antibody drugs, and belongs to the technical field of proteomics. According to the technical scheme, a specific peptide fragment obtained by pancreatin hydrolysis of Bevacizumab is selected, wherein specificity means that the peptide fragment is only generated by pancreatin hydrolysis of Bevacizumab, and other proteins and protein drugs cannot generate the peptide fragment by pancreatin hydrolysis; and based on liquid chromatography-tandem mass spectrometry technology, a parallel reaction monitoring modeis adopted to scan and analyze the specific peptide fragment to establish a stable and reliable LC / MS / MS quantitative method for the specific peptide fragment. The pharmacokinetic mass spectrometry analysis method of the monoclonal antibody drugs has the characteristics of high flux, high sensitivity, good reproducibility and the like. Reagents and drugs used are cheaper and easier to obtain, thepreparation method is simple, and the method is beneficial to popularization. A sample treatment process and quantitative method are simple and easy to implement, and guiding significance for qualitative and quantitative research of other protein drugs and polypeptide drugs is achieved.
Owner:南京智谱分子医学技术研究院有限公司
Microneedle array for rapid transdermal delivery of protein drugs and preparation method of microneedle array
InactiveCN111450403ARapid transdermal deliveryTransdermal Delivery SafePeptide/protein ingredientsMicroneedlesPolyelectrolyteSubcutaneous injection
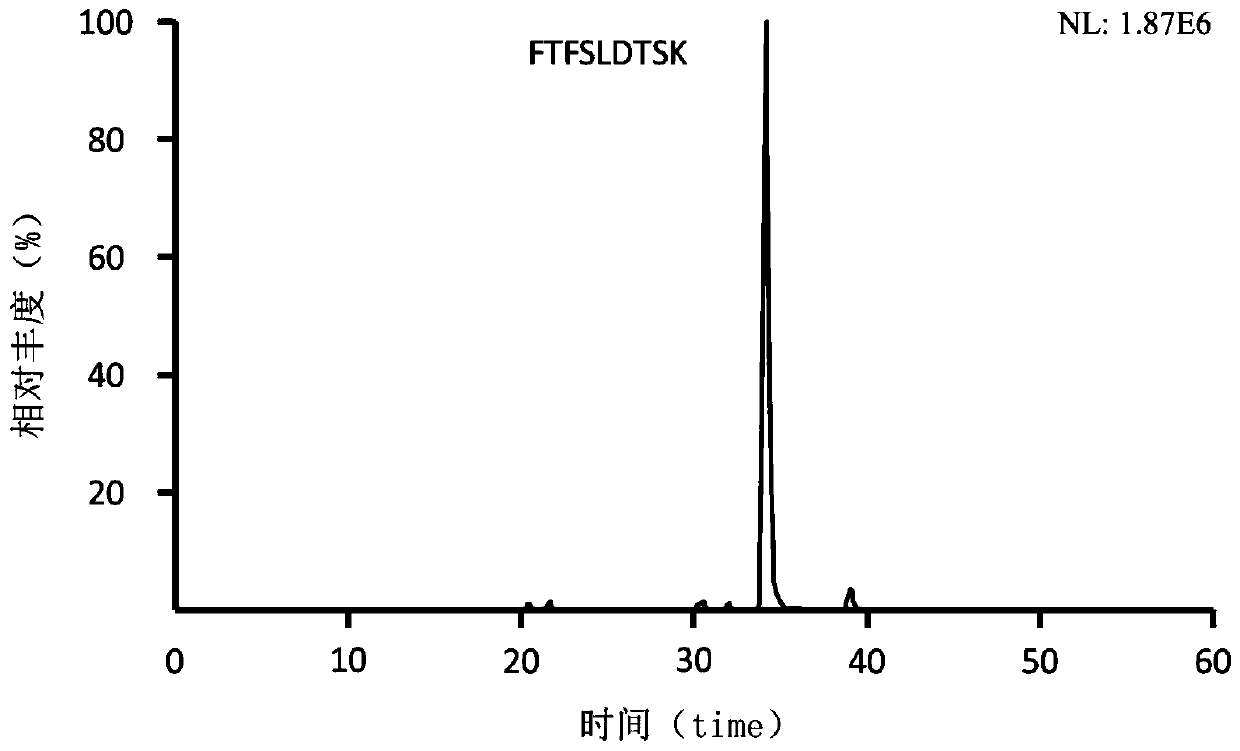
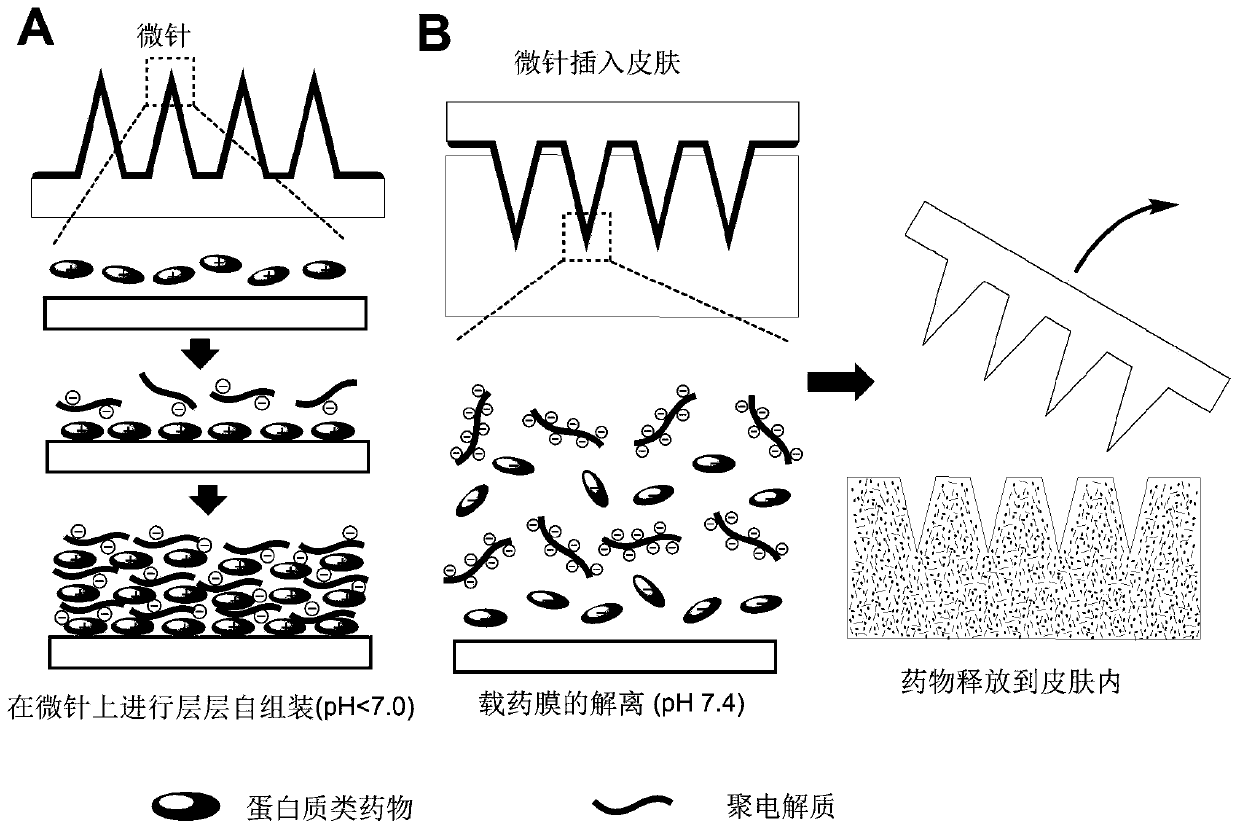
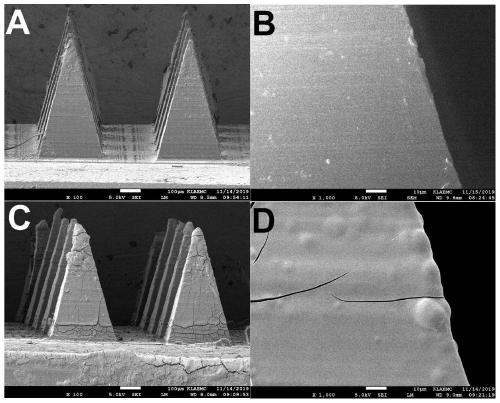
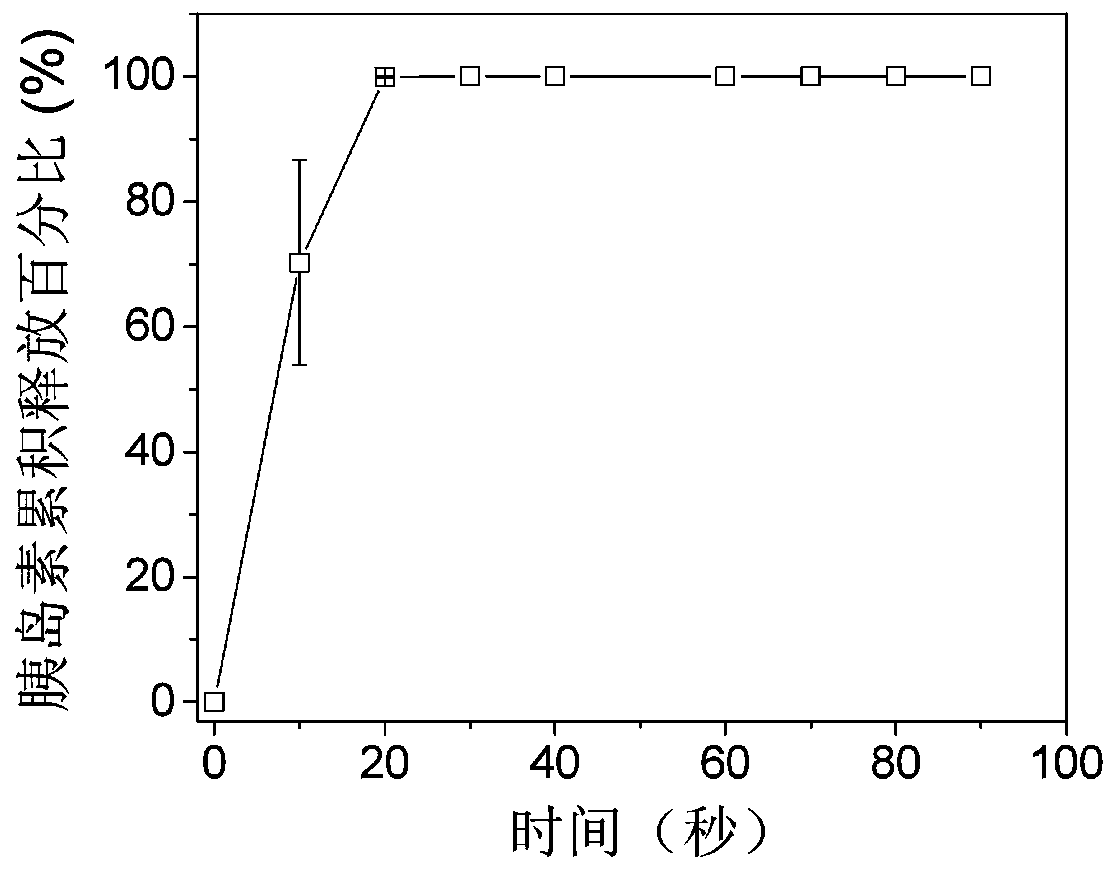
The invention provides a microneedle array for rapid transdermal delivery of protein drugs and a preparation method of the microneedle array, and belongs to the technical field of biomedicine. The structure of the microneedle array comprises a microneedle array substrate and a drug-loaded release layer deposited on the surface of a microneedle, and the drug-loaded release layer consists of a layer-by-layer self-assembled film formed by electrostatic interaction of the protein drugs and anionic polyelectrolyte. The microneedle array prepared by the method is simple and convenient to use, the application time is short and is only 10 seconds to 1 minutes, the microneedle array is taken away after application, self-administration of a patient is facilitated, the microneedle array has the advantages of being minimally invasive and painless, and compliance of the patient can be improved. The drugs are released quickly, and pharmacokinetics and pharmacodynamics equivalent to those of common subcutaneous injection can be obtained.
Owner:NANKAI UNIV
Rectal suppository of protein medicines and its preparing method and use in systemic disease therapy
InactiveCN1899604AAchieve systemic therapeutic effectPeptide/protein ingredientsDigestive systemDrugAnus
The present invention relates to a kind of protein medicine suppository administrated locally through anus and rectum to reach systemic treatment and its preparation process. The preparation process of the suppository includes mixing matrix component and other supplementary material in certain ratio, high pressure sterilizing at 121deg.c for 30 min and adding protein medicine at the temperature of 40+ / -5deg.c, cooling, molding, de-molding and packing. The present invention features that by means of the improved suppository preparation, it is possible to administrate protein medicine through anus and rectum for treating systemic disease.
Owner:CHANGCHUN GENESCIENCE PHARM CO LTD
TGase-activating protease inhibitor producing engineering bacteria and construction method thereof
ActiveCN102080064ASimple production processIncrease productionBacteriaMicroorganism based processesEscherichia coliStreptomyces hygroscopicus
The invention discloses TGase-activating protease inhibitor (TAPI) producing engineering bacteria and a construction method thereof and belongs to the field of genetic engineering. In the invention, the complete gene sequence of TAPI in Streptomyceshygroscopicus is obtained for the first time by a molecular biological means, a recombinant plasmid pET22b(+)-TAPI is constructed according to the obtained sequence, the TAPI is expressed in Escherichia coli, and when the bacteria are used for producing TAPI, the yield can reach 10mg / L. The method has the advantages of simple process, high product yield and the like, and a safe, mild and green novel protein surfactant is developed; and the target protein produced by the strain can be secreted outside cells directly, so it is easy to separate and purify the target protein. The invention provides a reference and basis for the genetic expression of protein surfactants and exploits a new direction for the development of the protein surfactants.
Owner:TAIXING DONGSHENG FOOD TECH
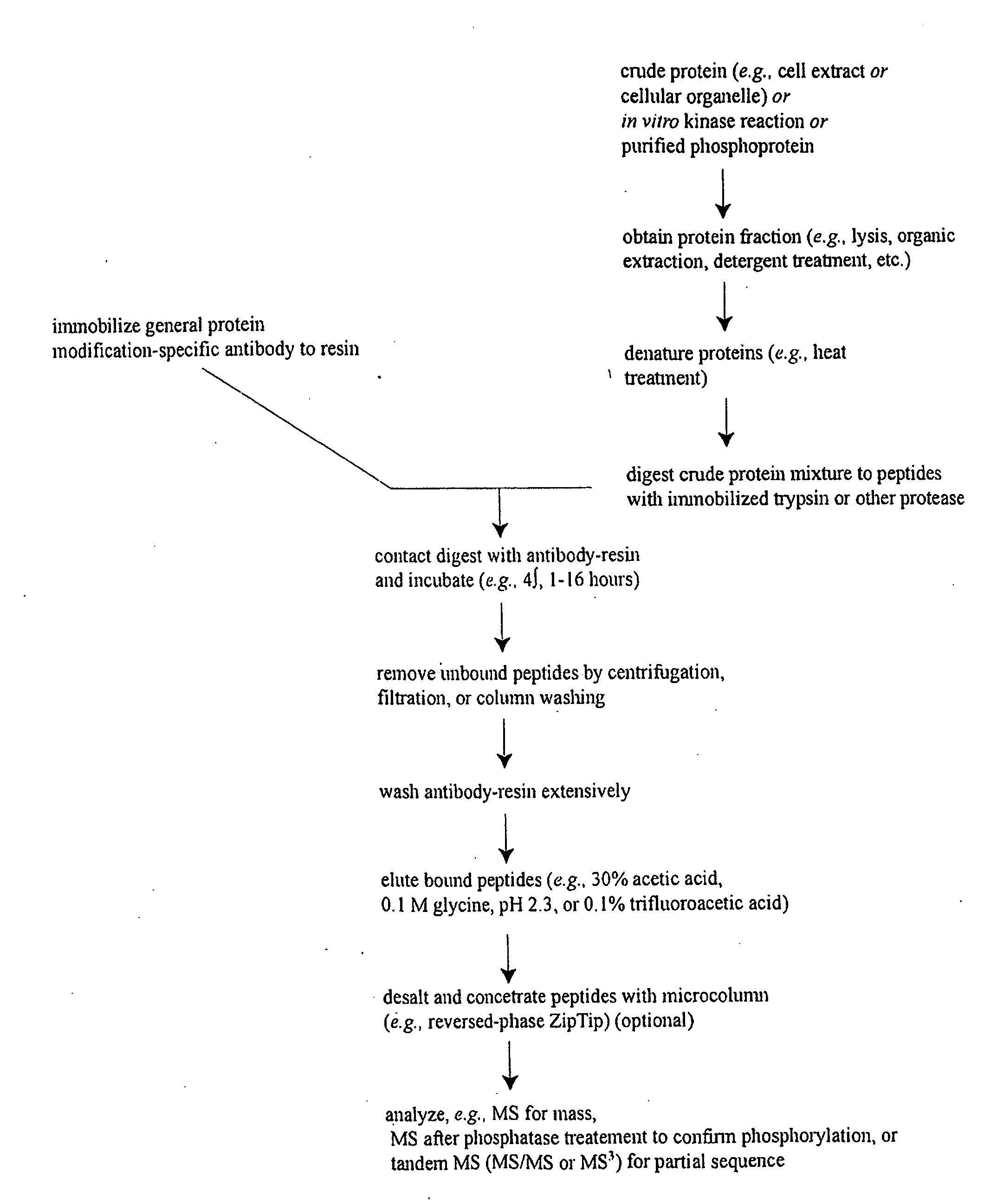
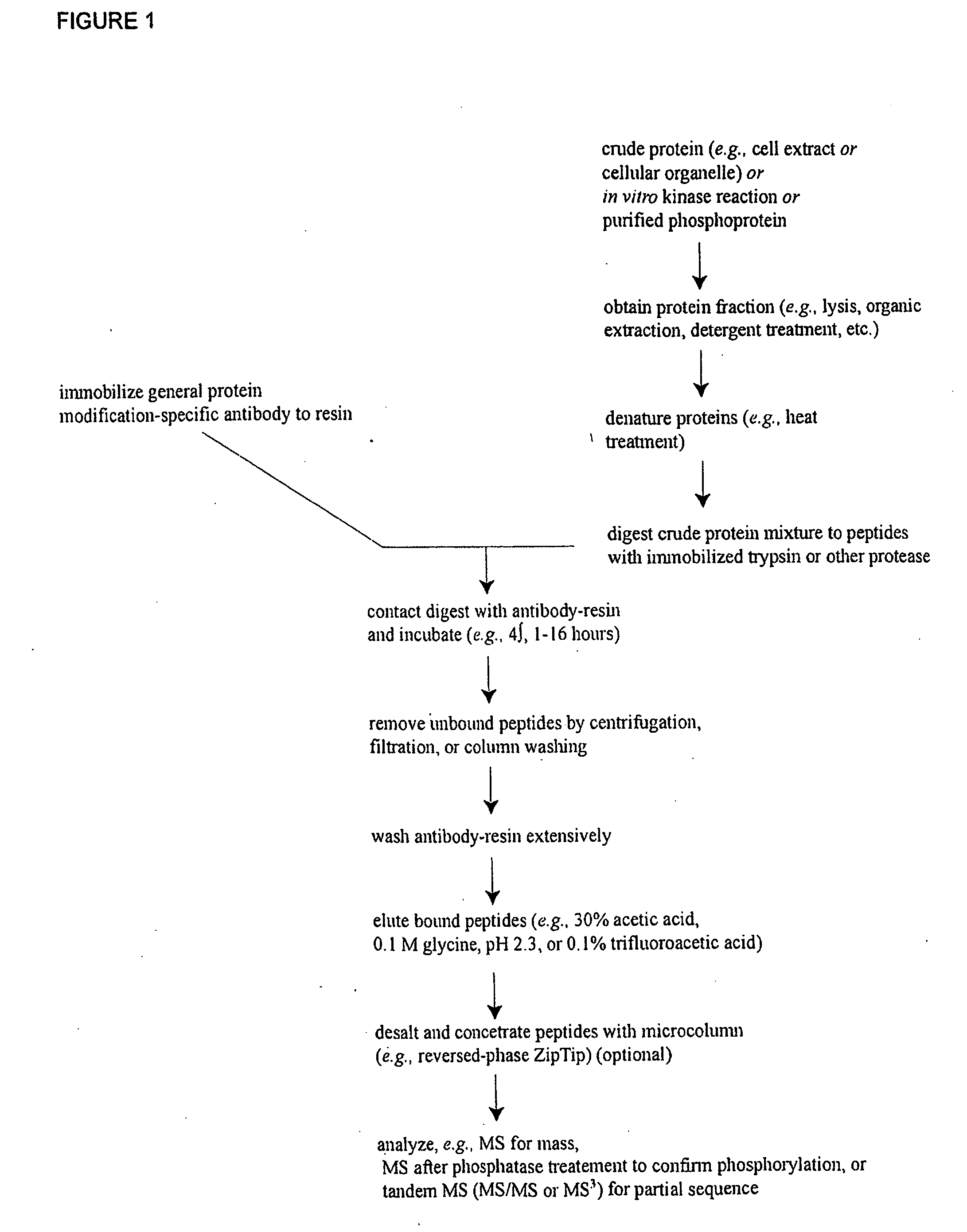
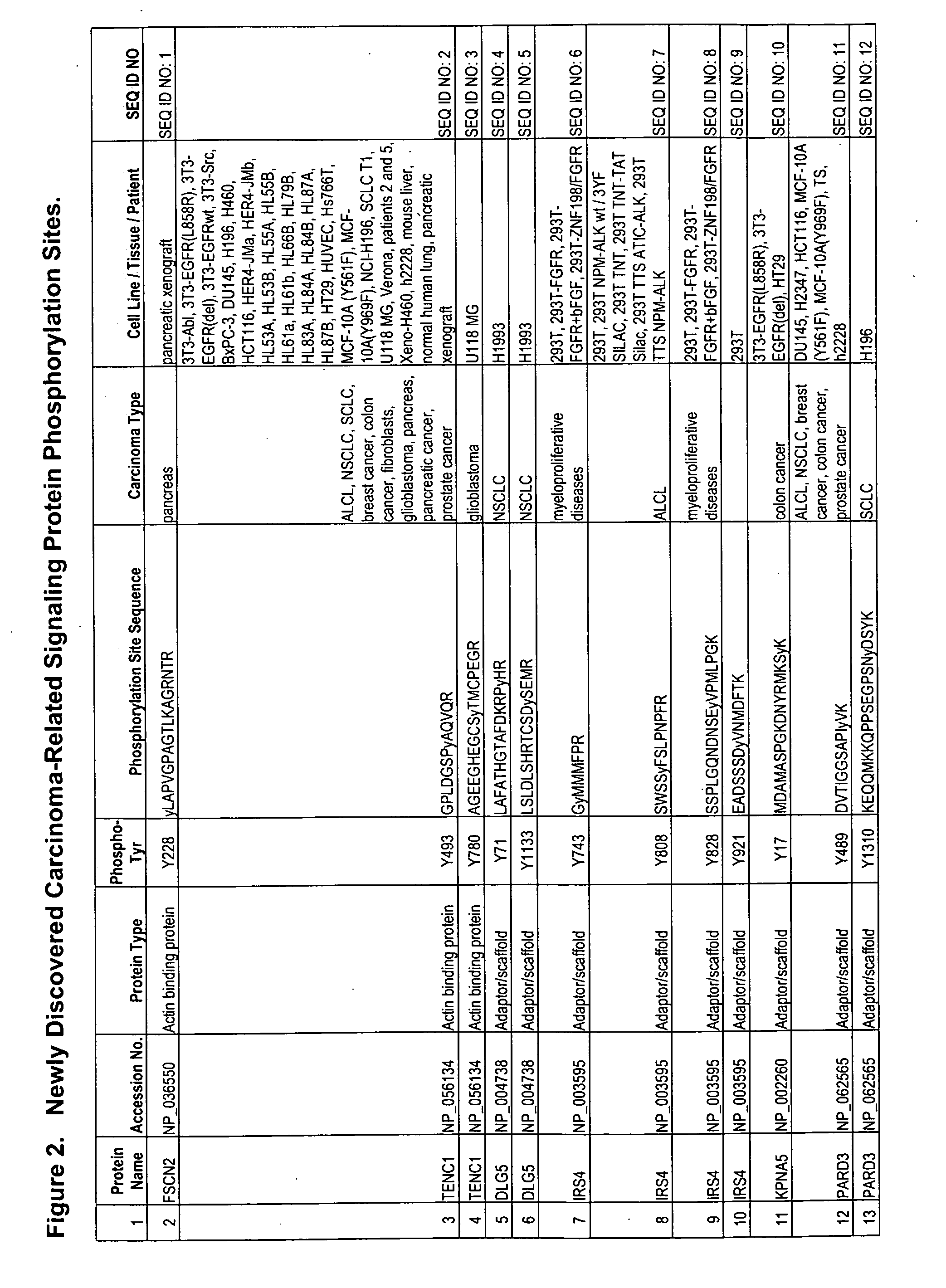
Reagents for the detection of protein phosphorylation in carcinoma signaling pathways
The invention discloses nearly 443 novel phosphorylation sites identified in signal transduction proteins and pathways underlying human carcinoma, and provides phosphorylation-site specific antibodies and heavy-isotope labeled peptides (AQUA peptides) for the selective detection and quantification of these phosphorylated sites / proteins, as well as methods of using the reagents for such purpose. Among the phosphorylation sites identified are sites occurring in the following protein types: Protein kinases (including Serine / Threonine dual specificity, and Tyrosine kinases), Adaptor / Scaffold proteins, Transcription factors, Phospoatases, Tumor supressors, Ubiquitin conjugating system proteins, Translation initiation complex proteins, RNA binding proteins, Apoptosis proteins, Adhesion proteins, G protein regulators / GTPase activating protein / Guanine nucleotide exchange factor proteins, and DNA binding / replication / repair proteins, as well as other protein types.
Owner:CELL SIGNALING TECHNOLOGY
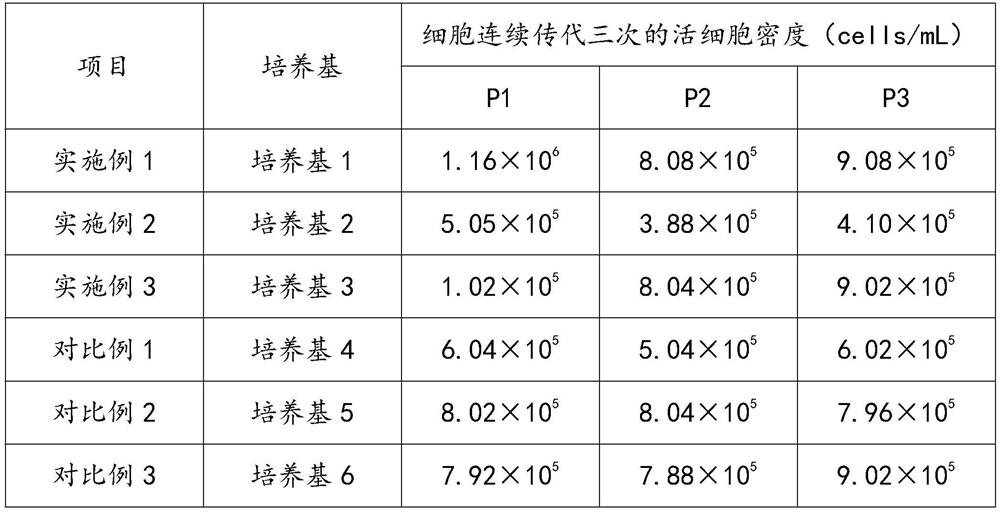
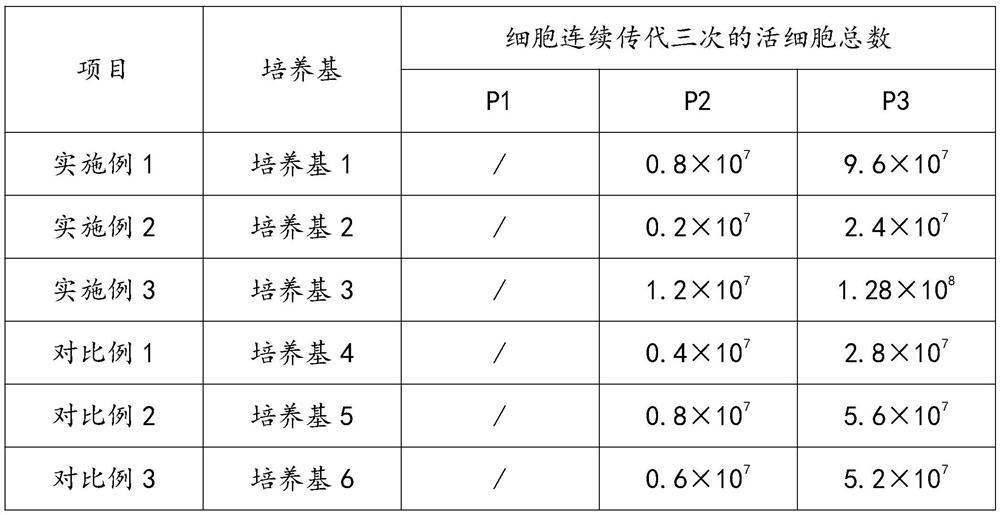
Serum-free culture medium for culturing Vero cells and preparation method of serum-free culture medium
PendingCN114540277AClear chemical compositionLow costCulture processArtificial cell constructsViral VaccineTrypsin inhibitor
The invention discloses a serum-free culture medium for culturing Vero cells and a preparation method thereof, the serum-free culture medium comprises a basic culture medium, the basic culture medium comprises glucose and glutamine, and the serum-free culture medium also comprises a main addition factor and a secondary addition factor, the main addition factors comprise proteins, hormones, growth factors, adherence promoting factors, trace elements and vitamins, and the secondary addition factors comprise a reducing agent, a trypsin inhibitor and a shear force protective agent. According to the culture medium, the Vero cells are cultured through the culture medium, normal growth of the Vero cells can be maintained, the amplification efficiency of the Vero cells can be improved, research, development and production of new coronavirus vaccines can be accelerated, the culture medium is clear in chemical component, free of serum and low in cost, biological safety risks caused by using serum are avoided, and the culture medium is suitable for popularization and application. The stability of a biological product production process and finished product quality is favorably improved.
Owner:杭州荣泽生物科技集团有限公司
Medical nutrition powder for patients suffering from nephropathy without dialysis, and preparation method of medical nutrition powder
InactiveCN111165802APromote absorptionAbsorption hasFood ingredient as thickening agentVitamin food ingredientsNutritionMedical nutrition
The invention discloses medical nutrition powder for patients suffering from nephropathy without dialysis, and a preparation method of the medical nutrition powder, and relates to the technical fieldof medical foods. The medical nutrition powder for patients suffering from nephropathy without dialysis comprises the following components in parts by weight of 52.5-420 parts of protein type substances, 87-696 parts of fat type substances, 263.5-2108 parts of carbohydrate, 7.95-63.6 parts of composite mineral substances, 4.17-33.36 parts of complex vitamins, 0.4-3.2 parts of taurine, 0.5-4 partsof mono and diglycerides of fatty acids, 0.09-0.7 part of nucleotide, 0.06-0.48 part of L-carnitine, 9-72 parts of composite thickening agents, 1-8 parts of vanilla essence and 0.28-2.2 parts of aspartame, wherein the carbohydrate contains 194.5-1556 parts of maltodextrin, 15-120 parts of inulin and 5-40 parts of crystallization levulose. The medical nutrition powder disclosed by the invention adopts a low-protein formula, besides, the used protein is high-quality protein, whey protein contributes to intestinal tract absorption and has the effect of resisting oxidation, and soy protein isolatecan retard the descending level of glomerular filtration rate and retard the disease process of chronic nephropathy patients.
Owner:特康药业集团有限公司
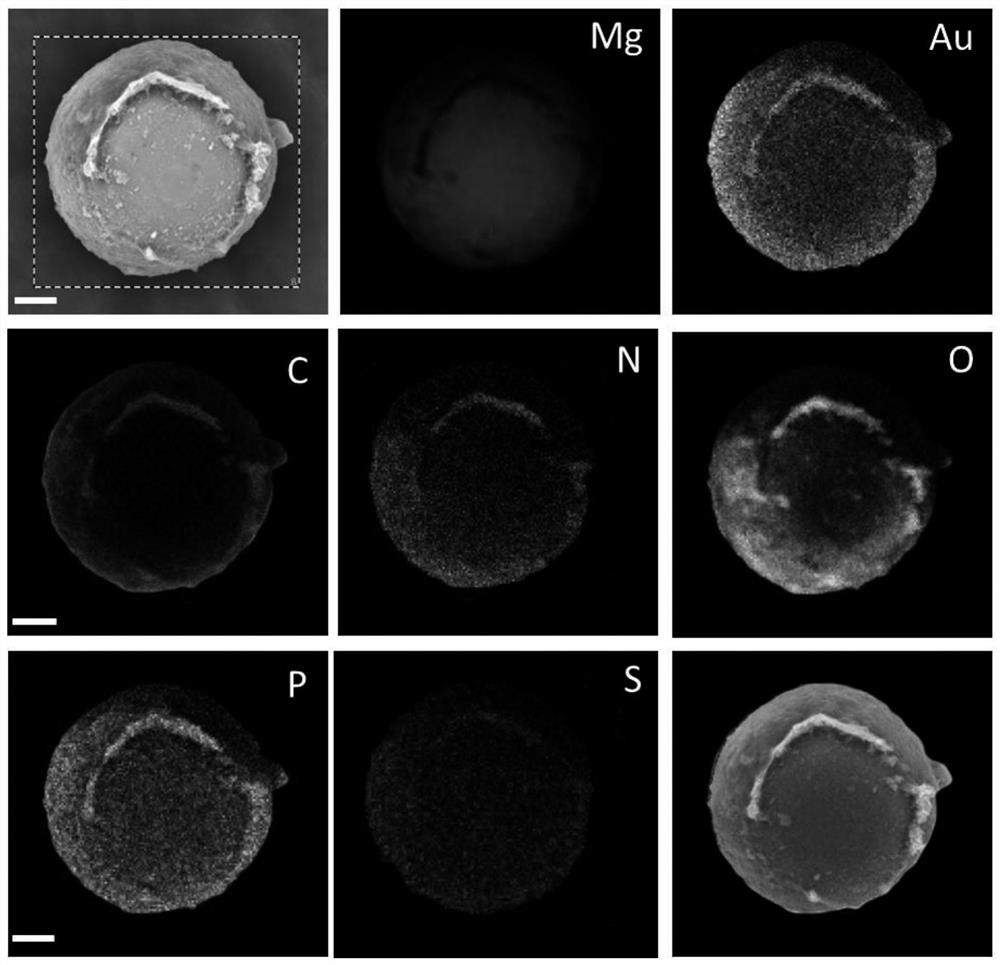
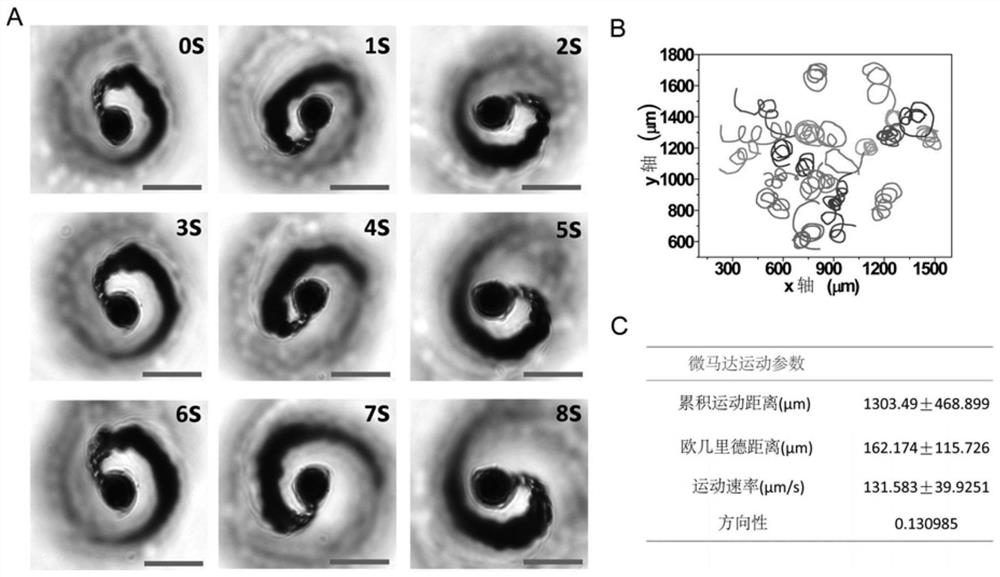
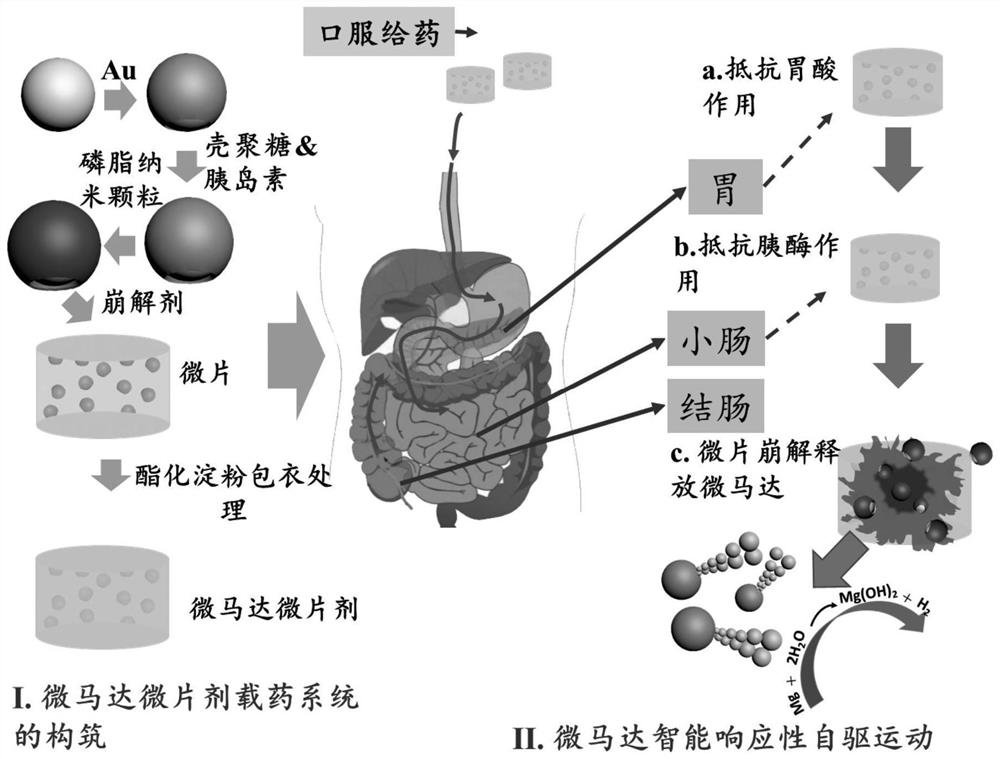
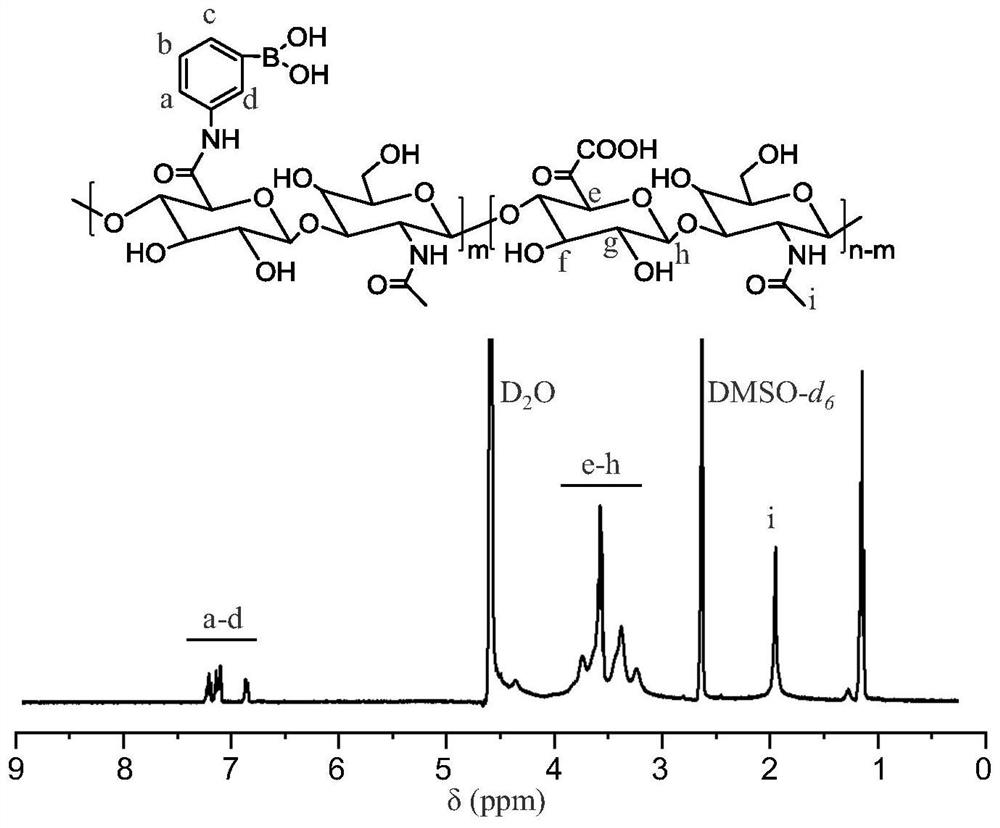
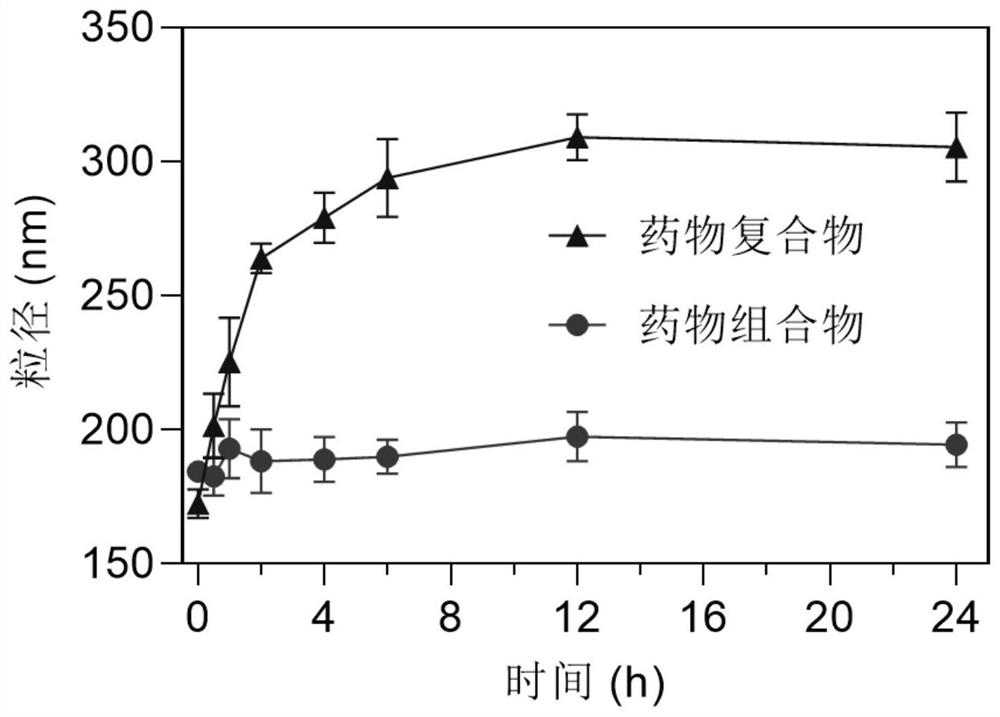
Micromotor carrier and preparation method and application thereof
ActiveCN113499321AHas colon-targeting propertiesImprove delivery efficiencyPeptide/protein ingredientsInorganic non-active ingredientsPhospholipinChitosan coating
The invention belongs to the technical field of micromotors, and discloses a micromotor carrier and a preparation method and application thereof. The micromotor carrier comprises a magnesium-based micromotor, an Au coating layer, a chitosan coating, a phospholipid layer and an esterified starch layer from inside to outside. The micromotor carrier is a self-driven micromotor, has a good colon targeting characteristic, is suitable for being used as an oral administration carrier of drugs, especially polypeptide and protein drugs, and can improve the contact probability of colon and the infiltration capacity of the colon to intestinal mucosa, so that the acceptable daily intake and bioavailability of the colon part to the drugs are improved.
Owner:SOUTHERN MEDICAL UNIVERSITY
Anti-inflammatory and antioxidant pharmaceutical composition as well as preparation method and application thereof
ActiveCN114748433AQuick fixReduced enrichmentPowder deliveryOrganic active ingredientsAminophenylboronic acidDrug administration
The invention discloses an anti-inflammatory and anti-oxidation pharmaceutical composition and a preparation method and application thereof.The pharmaceutical composition is composed of a nano-drug carrier and a drug compound entrapped by the nano-drug carrier, and the drug compound is obtained by non-covalent bond polymerization of polyphenols and protein drugs, the nano-drug carrier is hyaluronic acid modified by 3-aminophenylboronic acid. According to the pharmaceutical composition designed by the invention, the activity of a functional protein drug is protected through the nano-drug carrier, inflammatory tissues are recognized in a targeted manner, high-concentration active oxygen at an inflammatory part is responded, and protein drugs and polyphenol substances are released, so that accurate and efficient drug administration at an injured part can be realized, and meanwhile, anti-inflammatory and anti-oxidation synergistic curative effects are exerted; and the compound has good biological safety, and has a good application prospect in the aspect of preparing anti-inflammatory and anti-oxidation drugs.
Owner:SUZHOU UNIV
Plant derived antibiotic peptide rich in glycine
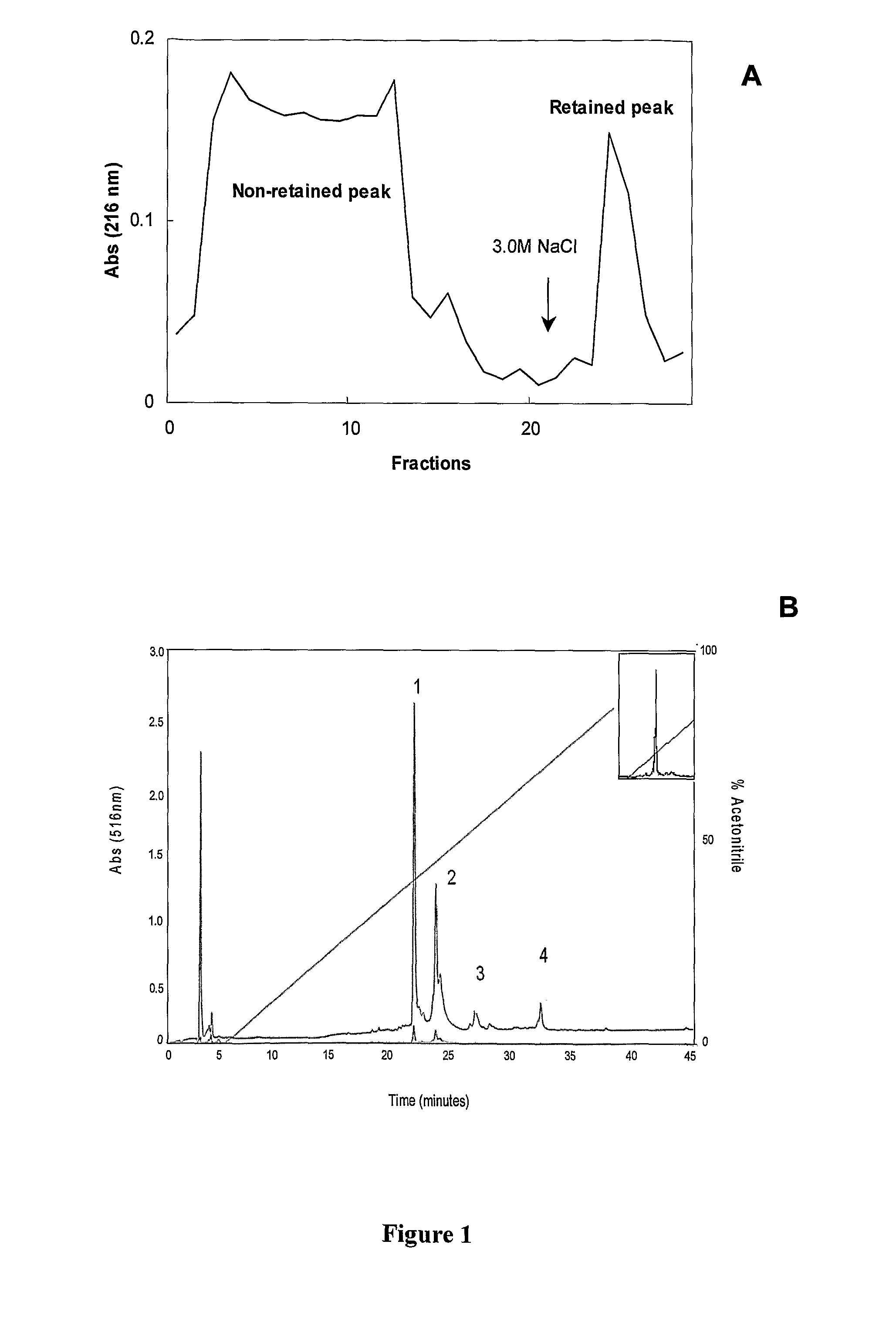
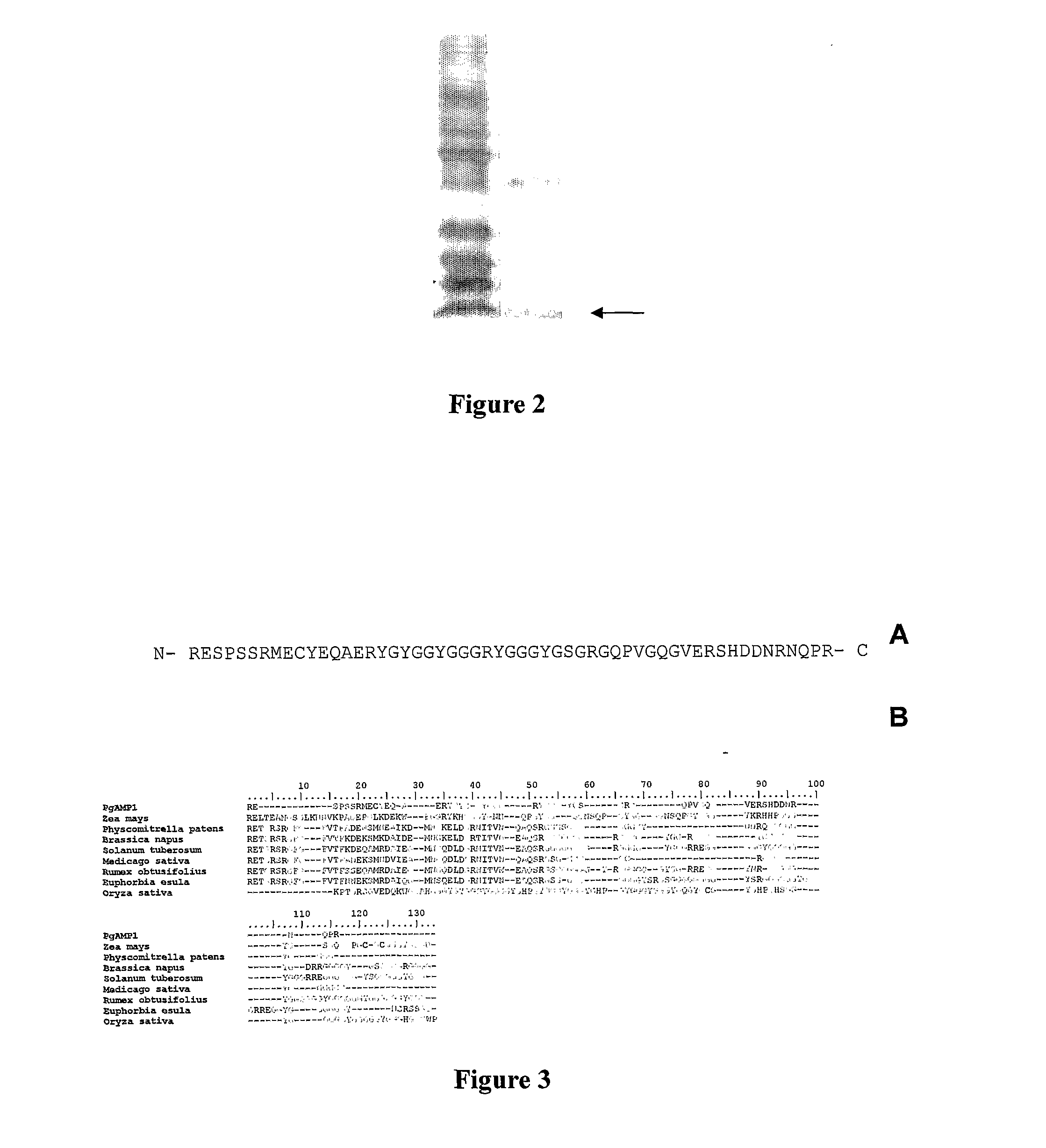
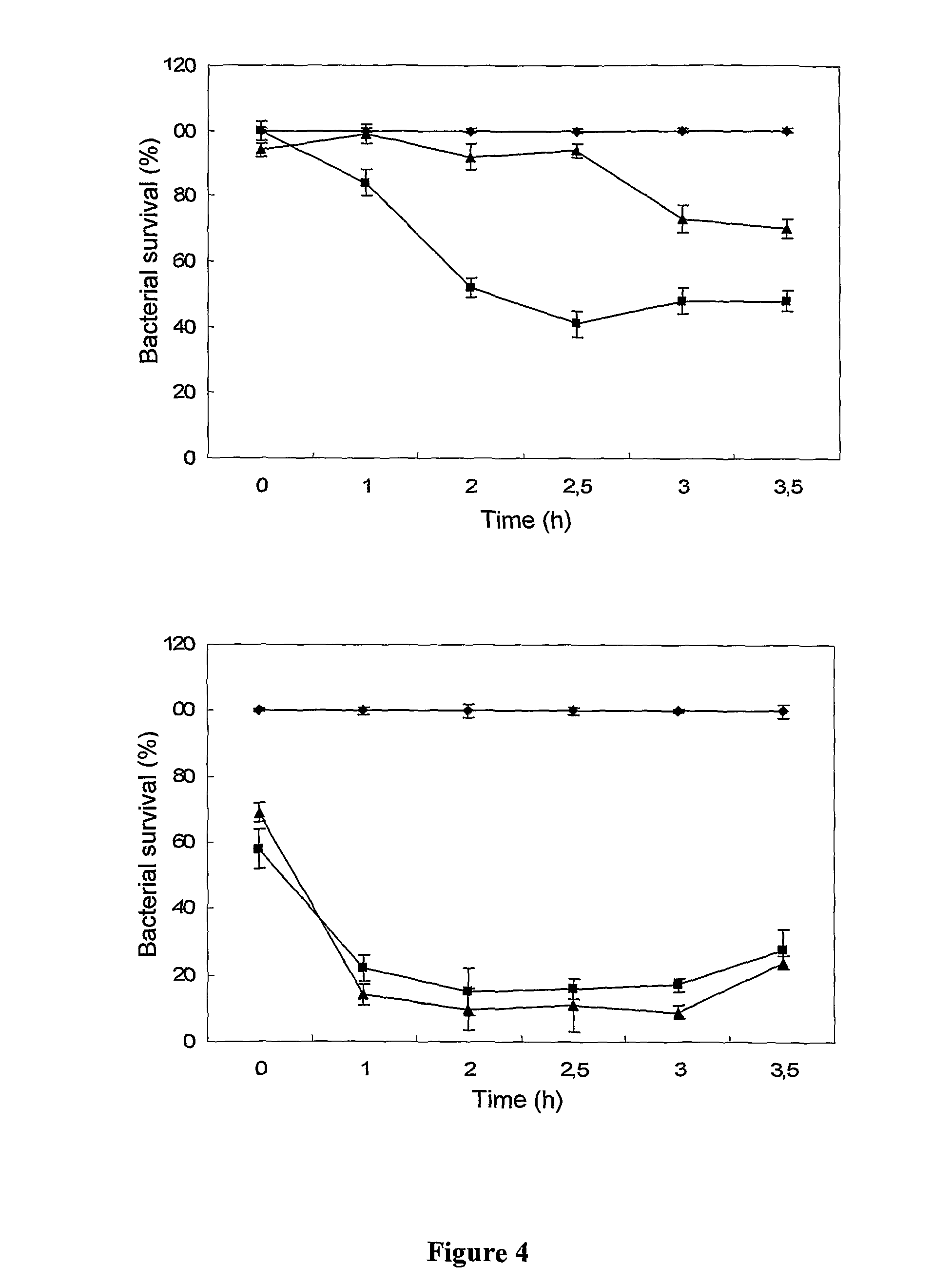
The present invention relates to a novel peptide extracted from guava (Psidium guajava) seeds, that provides bactericide activity, Preferentially against Gram-negative bacteria which are known to cause urinary, hospital, and intestinal tract infections (Proteus sp. And Klebsiella sp.). The peptide, that has the amino acid sequence RESPSSRMEC YEQAERYGYG GYGGGRYGGG YGSGRGQPVG QGVERSHDDN RNQPR, belongs to the class of glycine rich proteins and has approximately 5 kDa of molecular weight. The invention also relates to antibiotic compositions for human, veterinary and plant treatments. Alternatively, the peptide, or a functionally similar derivative, subjects of the present invention, can be used for transforming organisms aiming pathogen resistance, other adaptive advantages, as well as various properties, specially for plants and animals.
Owner:UNIAO BRASILEIRA DE EDUCACAO CATOLICA UBEC +1
Protein expression method
The invention provides a protein expression method. A serum-free animal-origin-free culture medium is used, a basic culture medium and a supplementary culture medium are adopted for large-scale production and expression of protein drugs, the basic culture medium is suitable for cell amplification and perfusion culture, and the supplementary culture medium is suitable for supplementary batch culture. The protein drug expressed by the method does not contain non-human glycosylation.
Owner:SHANGHAI SINOMAB BIOTECHNOLOGY CO LTD +2
A method for identifying specific peptides of protein-containing traditional Chinese medicine
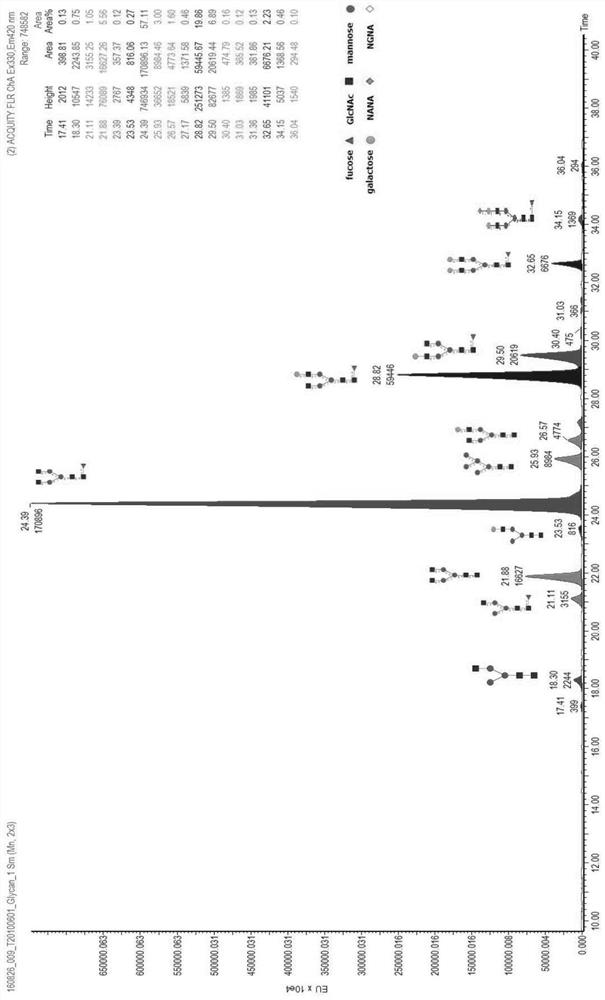
The invention discloses a method for identifying specific peptides of a Chinese medicine containing protein. The method comprises the following steps of: screening through a large number of experiments; taking a sample and performing enzymatic hydrolysis by using preferred enzyme; performing a desalination treatment; performing a high-throughput identification on peptide sequences using the best Nano LC-MS / MS method; verifying specific peptides by comparing differences in homologous protein sequences between different species of different samples; validating the specificity of the specific peptides in authentic products using the preferred LC-QQQ MS analysis. The method is scientific and easy to operate, and can be used to identify authentic and fake products of Chinese medicine, animal drugs, foods and aquatic products, and has important application value.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Soluble microneedle patch and preparation method thereof
PendingCN114306917AImprove bioavailabilityIncrease biological transdermalMedical devicesPolythylene glycolHydroxystearic Acid
The invention relates to a soluble microneedle patch and a preparation method thereof. The soluble microneedle patch comprises a substrate and a needle body on the substrate, the needle body comprises the following components: a high-molecular polymer framework material, polypeptide or protein drugs, a penetration enhancer and a stabilizer; the substrate comprises the high-molecular polymer framework material; the penetration enhancer is at least one of 15-hydroxystearic acid polyethylene glycol ester, Tween 80, deoxycholate, nicotinamide and amino acid. The microneedle patch is used for delivering polypeptide or protein drugs, the intradermal release and permeation speed of the polypeptide or protein drugs is obviously improved, the concentration of the polypeptide or protein drugs entering blood circulation is obviously increased, and the in-vivo bioavailability of the polypeptide or protein drugs after being delivered and administered through the microneedle is improved.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Protein expression method
The invention provides a protein expression method. A serum-free animal-origin-free culture medium is used, a basic culture medium and a supplementary culture medium are adopted for large-scale production and expression of protein drugs, the basic culture medium is suitable for cell amplification and perfusion culture, and the supplementary culture medium is suitable for supplementary batch culture. The protein drug expressed by the method does not contain non-human glycosylation.
Owner:SHANGHAI BIOMABS PHARMA +2
TGase-activating protease inhibitor producing engineering bacteria and construction method thereof
ActiveCN102080064BSimple production processIncrease productionBacteriaMicroorganism based processesEscherichia coliStreptomyces hygroscopicus
The invention discloses TGase-activating protease inhibitor (TAPI) producing engineering bacteria and a construction method thereof and belongs to the field of genetic engineering. In the invention, the complete gene sequence of TAPI in Streptomyceshygroscopicus is obtained for the first time by a molecular biological means, a recombinant plasmid pET22b(+)-TAPI is constructed according to the obtained sequence, the TAPI is expressed in Escherichia coli, and when the bacteria are used for producing TAPI, the yield can reach 10mg / L. The method has the advantages of simple process, high product yield and the like, and a safe, mild and green novel protein surfactant is developed; and the target protein produced by the strain can be secreted outside cells directly, so it is easy to separate and purify the target protein. The invention provides a reference and basis for the genetic expression of protein surfactants and exploits a new direction for the development of the protein surfactants.
Owner:TAIXING DONGSHENG FOOD TECH
Linker, drug-loaded linker, cell-penetrating peptide-coupled drug, antibody-coupled drug and preparation method thereof
ActiveCN110590877BImprove stabilityLittle side effectsSugar derivativesPharmaceutical non-active ingredientsDrug conjugationPharmaceutical Substances
The invention relates to the field of drug delivery, and discloses a linker, a drug-loaded linker, a cell-penetrating peptide-coupled drug, an antibody-coupled drug and a preparation method thereof. The linker has a structure shown in formula (I), and the invention provides The light-responsive self-catalytic cleavage linker and the drug-loaded linker can couple the drug to the polypeptide or protein delivery carrier, and can realize the controllable delivery of the drug into the cell.
Owner:NANKAI UNIV
A kind of alendronic acid-functionalized polyethylene glycol-modified nanoparticles and preparation method thereof
ActiveCN104147614BExtension of timeLow costGenetic material ingredientsPharmaceutical non-active ingredientsPolythylene glycolProtein
Owner:SHENZHEN INST OF ADVANCED TECH
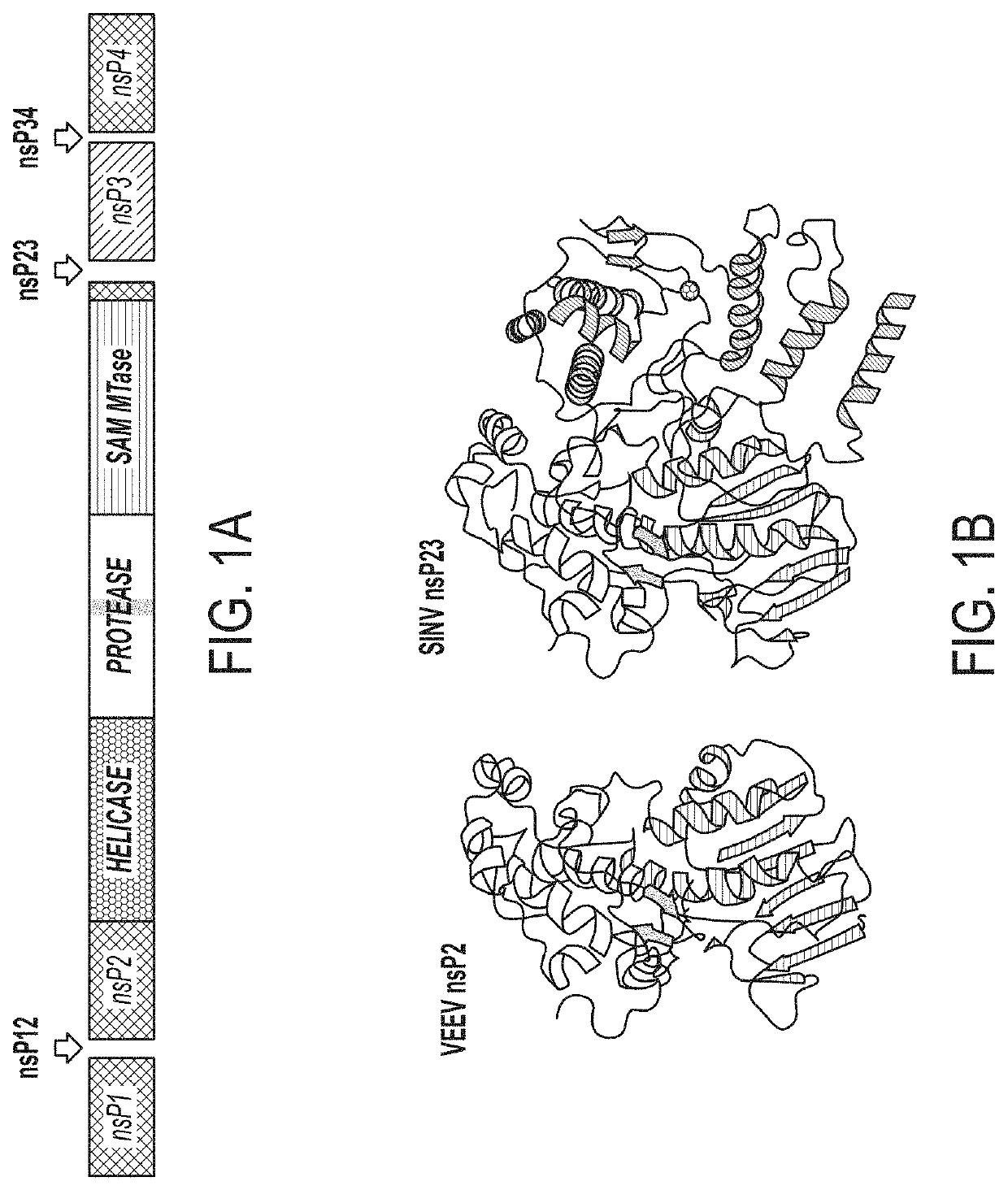
Methods and Compositions for the Detection of Host Protein Cleavage by Group IV Viral Proteases
PendingUS20210389324A1Loss of functionSsRNA viruses positive-senseHydrolasesPost translationalProtein
Proteases of Group IV (+)ssRNA viruses were found to act on a human sequences in addition to the viral sequences. The identity of the cleavable human sequences is disclosed. Detection of these sequences can act as a diagnostic of infection. It is contemplated that these findings could be employed to facilitate post-translational silencing at the level of protein (e.g., removal of existing proteins), thus serving as a protein analog to CRISPR / Cas9 and RNAi / RISC, and further to enable sequence-specific silencing of host functions without the modification of the host genome.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
A kind of serum miRNA marker and the method for detecting ionizing radiation damage thereof
ActiveCN109385472BStable extractionStable storageMicrobiological testing/measurementDNA/RNA fragmentationSerum mirnaProtein
The present invention relates to a serum miRNA marker, which is a combination of miR-134-5p and miR-155-5p. At the same time, the invention also discloses a method for detecting ionizing radiation damage by the serum miRNA marker. Compared with traditional chromosomal aberrations and protein biomarkers, the serum miRNA markers of the present invention are more stable in the extraction and storage process, making the detection results more reliable and accurate; and verified by qRT-PCR method, its expression level and radiation The dose is also closely related, which can provide a reliable and accurate assessment basis for judging whether the test object is at risk of being damaged by ionizing radiation.
Owner:INST OF MODERN PHYSICS CHINESE ACADEMY OF SCI
Environmental stimulation responsive protein polymer conjugate self-assembled body and preparation method and application thereof
ActiveCN110101868AImprove bioavailabilityReduced release ratePeptide/protein ingredientsMetabolism disorderHalf-lifeProtein polymer conjugates
The invention discloses an environmental stimulation responsive protein polymer conjugate self-assembled body and a preparation method and application thereof and belongs to the field of biomedicine.A protein polymer conjugate of the self-assembled body comprises a protein substance and two or more environmental stimulation responsive polymers coupled with the protein substance and is subjected to environmental stimulation sensitive responsive self-assembly under the action of temperature, enzymes, pH, light, static electricity, magnetic fields, chemical substances and other environments, a self-assembled body structure of the protein polymer conjugate can significantly improve the stability of drugs and effectively maintain the activity of the drugs, the half-life periods of the drugs are significantly prolonged at the same time, and pharmacokinetic parameters are improved; moreover, the preparation method is simple, condition parameters are easy to control, and the self-assembled body has a high practical value. The environmental stimulation responsive protein polymer conjugate self-assembled body has a broad application prospect in the fields of drug preparation and release control over the drugs.
Owner:PEKING UNIV
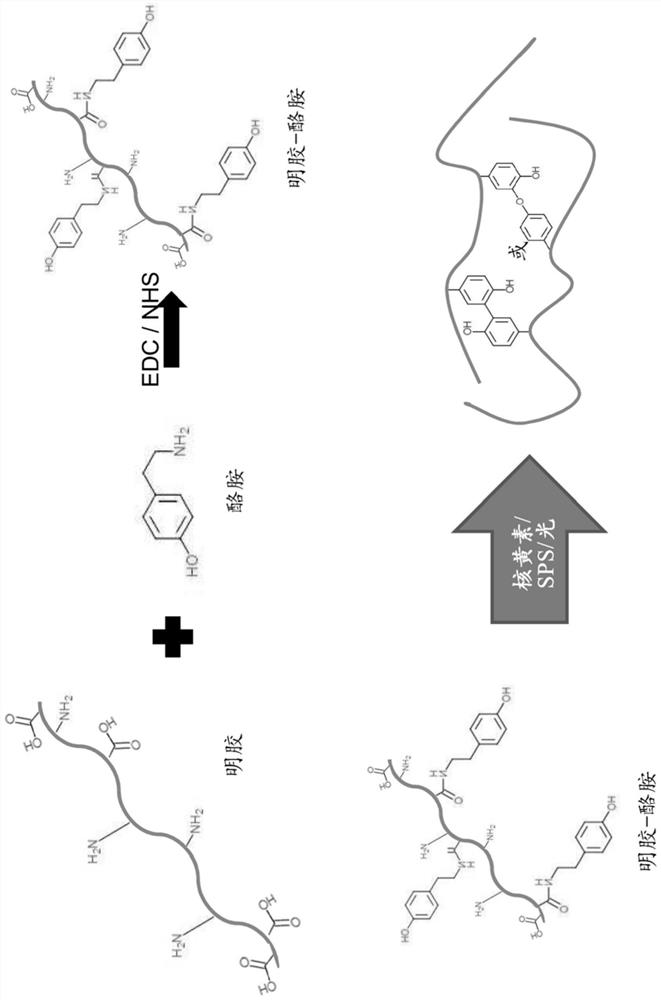
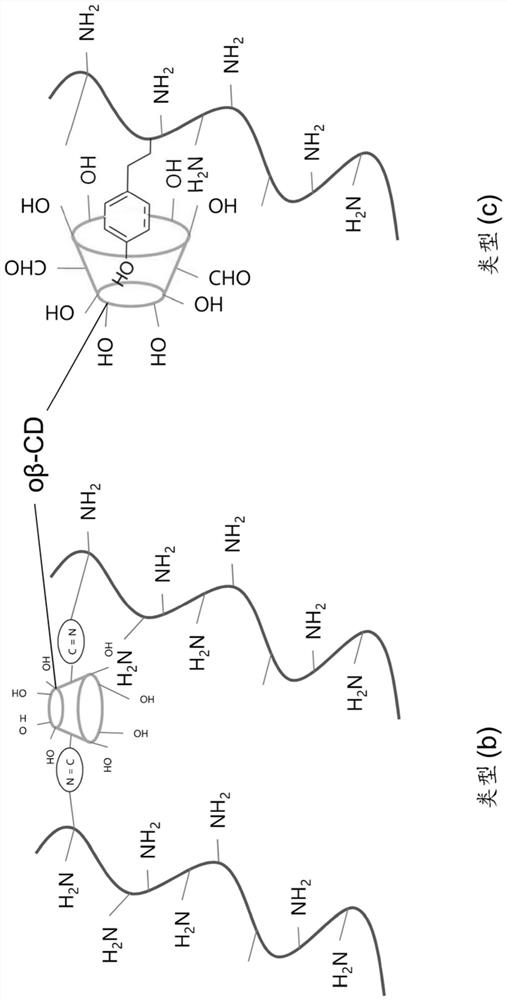
Hydrogel for in-vivo release of medication
The present invention relates to a hydrogel for in-vivo release of medication comprising at least one medication, wherein the hydrogel comprises (i) a protein-based biopolymer functionalized with a functionalisation agent that is able to form guest-host interactions with oxidized beta-cyclodextrin, preferably a primary aminoalkylphenol, more preferably gelatin functionalized with tyramine (GTA) and (ii) oxidized beta-cyclodextrin (o[beta]-CD), wherein the hydrogel is cross-linked via exposure to visible light in presence of a biocompatible photoinitiator, resulting in a degree of swelling in the range of 2-20 calculated as (swollen weight- dry weight) / dry weight. It further relates to a method for its preparation, as well as to a medication for treatment of musculoskeletal disorders, preferably for treatment of infection, inflammation, malignant processes, growth disorders, degenerative disorders or treatment of pain arising from (surgical treatment of) these disorders.
Owner:UMC UTRECHT HLDG BV +2
Protein expression method
The invention provides a protein expression method. A serum-free animal-origin-free culture medium is used, a basic culture medium and a supplementary culture medium are adopted for large-scale production and expression of protein drugs, the basic culture medium is suitable for cell amplification and perfusion culture, and the supplementary culture medium is suitable for supplementary batch culture. The protein drug expressed by the method does not contain non-human glycosylation.
Owner:SHANGHAI ZHANGJIANG BIOTECH CO LTD +2
Protein expression method
The invention provides a protein expression method. A serum-free animal-origin-free culture medium is used, a basic culture medium and a supplementary culture medium are adopted for large-scale production and expression of protein drugs, the basic culture medium is suitable for cell amplification and perfusion culture, and the supplementary culture medium is suitable for supplementary batch culture. The protein drug expressed by the method does not contain non-human glycosylation.
Owner:SHANGHAI ZHANGJIANG BIOTECH CO LTD +2
A self-assembly of environmental stimulus-responsive protein polymer conjugates and its preparation method and application
ActiveCN110101868BImprove bioavailabilityReduced release ratePeptide/protein ingredientsMetabolism disorderPharmaceutical drugDrugs preparations
The invention discloses an environmental stimulation responsive protein polymer conjugate self-assembled body and a preparation method and application thereof and belongs to the field of biomedicine.A protein polymer conjugate of the self-assembled body comprises a protein substance and two or more environmental stimulation responsive polymers coupled with the protein substance and is subjected to environmental stimulation sensitive responsive self-assembly under the action of temperature, enzymes, pH, light, static electricity, magnetic fields, chemical substances and other environments, a self-assembled body structure of the protein polymer conjugate can significantly improve the stability of drugs and effectively maintain the activity of the drugs, the half-life periods of the drugs are significantly prolonged at the same time, and pharmacokinetic parameters are improved; moreover, the preparation method is simple, condition parameters are easy to control, and the self-assembled body has a high practical value. The environmental stimulation responsive protein polymer conjugate self-assembled body has a broad application prospect in the fields of drug preparation and release control over the drugs.
Owner:PEKING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com