Hydrogel for in-vivo release of medication
A hydrogel and drug technology, applied in the direction of drug combination, drug delivery, prosthesis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0072] Use the method described in the instructions.
[0073] Material:
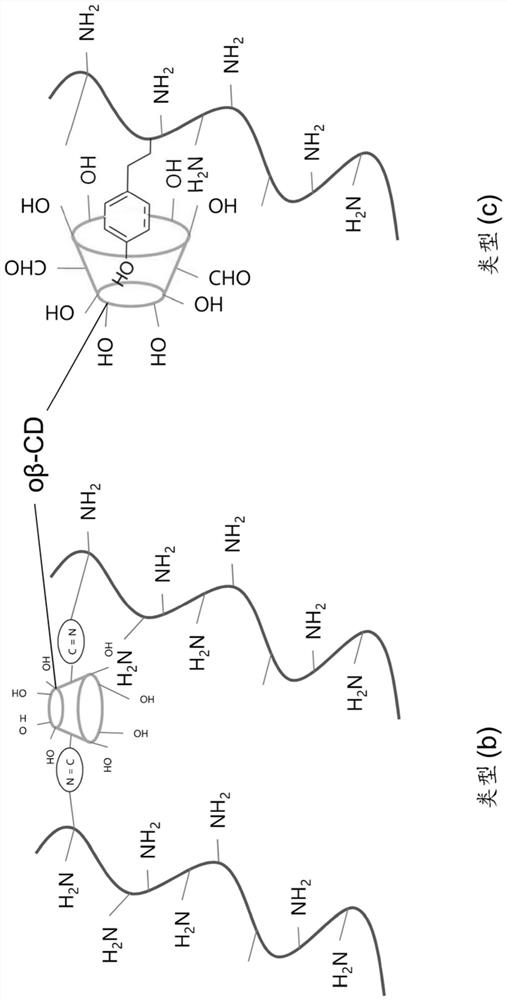
[0074] Gelatin (porcine skin, type A, 300 g gel strength), 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC), N-hydroxysuccinimide (NHS), Tyramine hydrochloride, 2-morpholineethanesulfonic acid monohydrate (MES), sodium persulfate (SPS), sodium bicarbonate (NaHCO 3 ), sodium periodate, β-cyclodextrin, glycerol, phosphate buffered saline (PBS), riboflavin (RB) and ethylene glycol were purchased from Sigma Aldrich. Cellulose Dialysis Membrane (Spectrum / Por TM , 0,5 kDa; 12 kDa molecular weight cut-off) was purchased from Spectrum Laboratories. Bupivacaine was obtained from Siegfried, Switzerland.
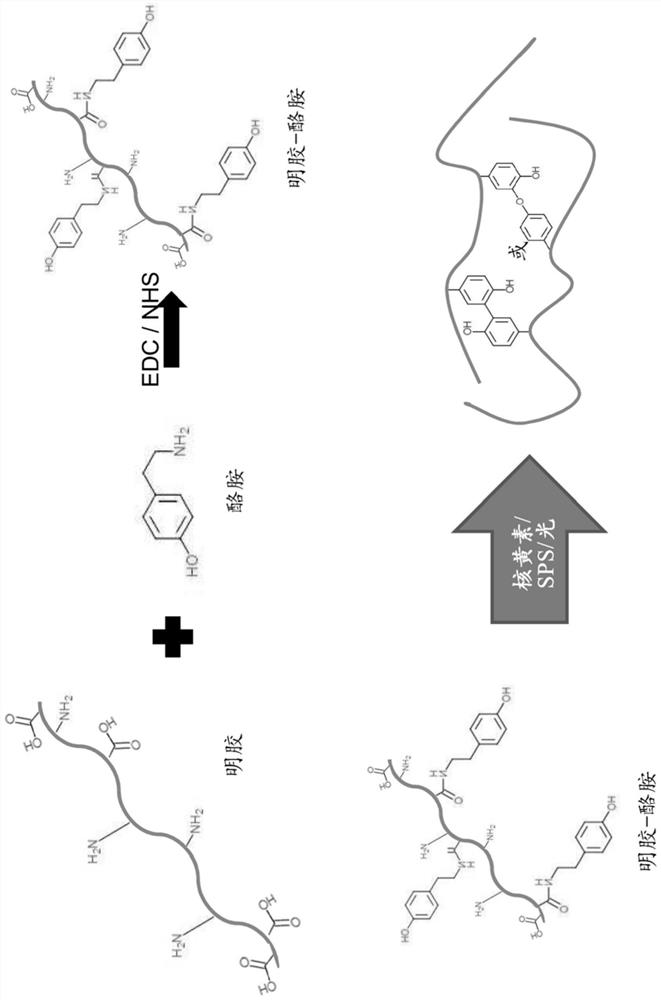
[0075] Synthesis of gelatin-tyramide (GTA):
[0076] Type A gelatin was dissolved in MES buffer at 50°C, then tyramide, EDC and NHS were added. The reaction mixture was stirred overnight. The mixture was then dialyzed against water and the product was obtained by lyophilization.
PUM
| Property | Measurement | Unit |
|---|---|---|
| elastic modulus | aaaaa | aaaaa |
| compression force | aaaaa | aaaaa |
| compressive modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com