Serum-free culture medium for culturing Vero cells and preparation method of serum-free culture medium
A serum-free medium and basal medium technology, applied in biochemical equipment and methods, culture process, tissue culture and other directions, can solve the problems of poor cell adherence, unknown composition, and unsatisfactory cell proliferation rate, and achieve low cost. , controllable between batches, and the effect of avoiding biosafety risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
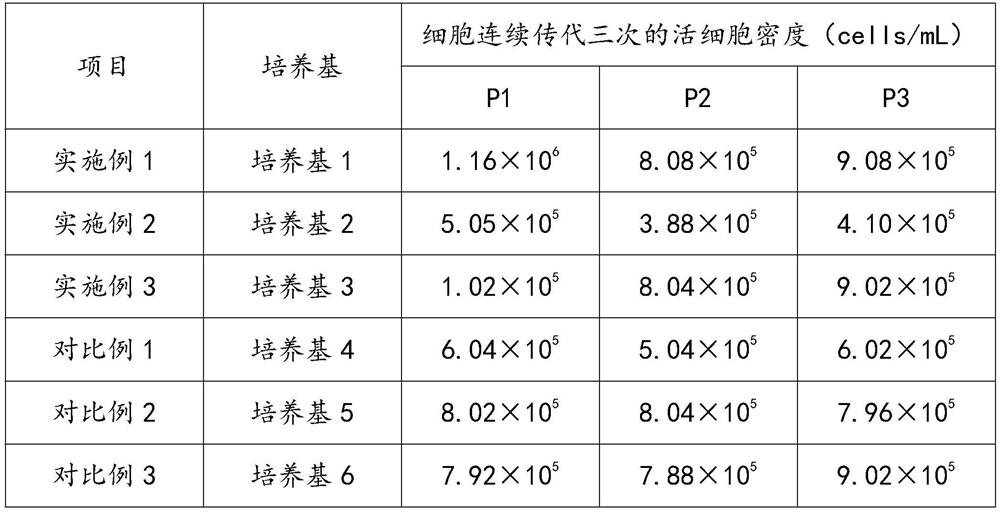
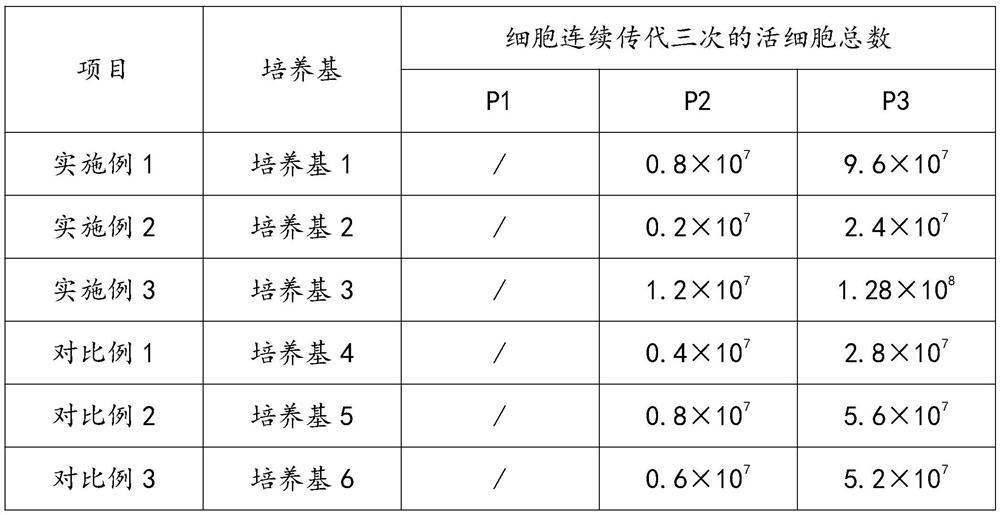
[0053] Example 1: Medium 1: basal medium, 800 mg / L vegetable hydrolyzed protein, 2.8 mg / L Fe 3+ -Sulfate-iminodiacetic acid complex, 7.2mg / L Fe 3+ -Sulfate-glycylglycine complex, 5mg / L insulin, 1.28mg / L auxin, 1.0mg / L hydrocortisone, 2.2mg / L cortisol, 0.01mg / L epidermal growth factor EGF, 0.6mg / L transforming growth factor TGF, 0.15mg / L platelet growth factor PDGF, 1.8mg / L fibroblast growth factor FGF, 2.5mg / L fibronectin, 1.4mg / L collagen, 0.3mg / L laminin, 1.1mg / L poly-L-lysine, 0.4mg / L ornithine, 0.01mg / L sodium selenite, 0.03mg / L gold tricarboxylic acid, 0.008mg / L Zn 2+ , 0.07mg / L Riboflavin, 0.22mg / L Folic Acid, 0.17mg / L Cobalamin, 0.1mg / L Calcium Pantothenate, 7.68mg / L Inositol, 0.3mg / L Niacinamide, 3.5mg / L Chloride Choline, 0.04mg / L lipoic acid, 0.02mg / L pyridoxal hydrochloride, 0.27mg / L vitamin C, 0.01mg / L vitamin E, 0.23mg / L pyridoxine hydrochloride, 0.005mg / L carboxylesterase , 301.44mg / L potassium chloride, 13mg / L calcium chloride, 118mg / L sodium dihydrogen phosp...
Embodiment 2
[0054] Example 2: Medium 2: basal medium, 500mg / L serum albumin, 15mg / L transferrin, 5mg / L insulin, 1.28mg / L auxin, 1.0mg / L hydrocortisone, 2.2mg / L L cortisol, 0.01mg / L epidermal growth factor EGF, 0.6mg / L transforming growth factor TGF, 0.15mg / L platelet growth factor PDGF, 1.8mg / L fibroblast growth factor FGF, 2.5mg / L fibronectin, 1.4mg / L collagen, 0.3mg / L laminin, 1.1mg / L poly-L-lysine, 0.4mg / L ornithine, 0.01mg / L sodium selenite, 0.03mg / L gold essence Tricarboxylic acid, 0.008mg / L Zn 2+ , 0.07mg / L Riboflavin, 0.22mg / L Folic Acid, 0.17mg / L Cobalamin, 0.1mg / L Calcium Pantothenate, 7.68mg / L Inositol, 0.3mg / L Niacinamide, 3.5mg / L Chloride Choline, 0.04mg / L lipoic acid, 0.02mg / L pyridoxal hydrochloride, 0.27mg / L vitamin C, 0.01mg / L vitamin E, 0.23mg / L pyridoxine hydrochloride, 0.005mg / L carboxylesterase , 301.44mg / L potassium chloride, 13mg / L calcium chloride, 118mg / L sodium dihydrogen phosphate, 6801mg / L sodium chloride, 68.835mg / L magnesium sulfate, 0.15mg / L linoleic acid, ...
Embodiment 3
[0055] Example 3: Medium 3: basal medium, 500mg / L serum albumin, 15mg / L transferrin, 5mg / L insulin, 1.28mg / L auxin, 1.0mg / L hydrocortisone, 2.2mg / L L cortisol, 0.01mg / L epidermal growth factor EGF, 0.6mg / L transforming growth factor TGF, 0.15mg / L platelet growth factor PDGF, 1.8mg / L fibroblast growth factor FGF, 2.5mg / L fibronectin, 1.4mg / L collagen, 0.3mg / L laminin, 1.1mg / L poly-L-lysine, 0.4mg / L ornithine, 0.01mg / L sodium selenite, 0.03mg / L gold essence Tricarboxylic acid, 0.008mg / L Zn 2+ , 0.07mg / L Riboflavin, 0.22mg / L Folic Acid, 0.17mg / L Cobalamin, 0.1mg / L Calcium Pantothenate, 7.68mg / L Inositol, 0.3mg / L Niacinamide, 3.5mg / L Chloride Choline, 0.04mg / L lipoic acid, 0.02mg / L pyridoxal hydrochloride, 0.27mg / L vitamin C, 0.01mg / L vitamin E, 0.23mg / L pyridoxine hydrochloride, 0.005mg / L carboxylesterase , 301.44mg / L potassium chloride, 13mg / L calcium chloride, 118mg / L sodium dihydrogen phosphate, 6801mg / L sodium chloride, 68.835mg / L magnesium sulfate, 1.2mg / L amphotericin B, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com