Recombinant human erythropoietin-CTP fusion protein production process and application
A technology of fusion protein and -CTP, which is applied in the direction of drug combination, peptide/protein composition, recombinant DNA technology, etc., can solve the problems of frequent administration of patients and short half-life of EPO in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 expression strain construction method
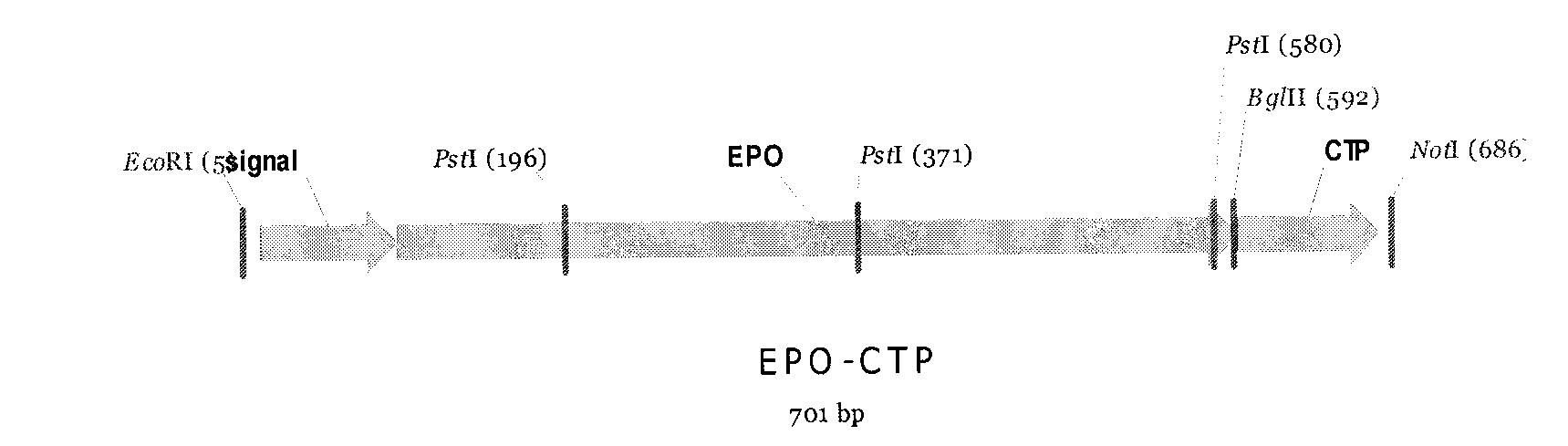
[0040] The EPO-CTP fusion protein gene that will be connected on the pMD18-T carrier, utilizes Not I and EcoR I double enzyme digestion to identify positive clone ( figure 1 ), sequenced. After obtaining positive clones with the correct sequence, the fusion gene was connected to the pcDNA eukaryotic expression vector, and the constructed expression strain could be transfected into CHO cells after extracting the plasmid. Schematic diagram of the structure of the EPO-CTP fusion protein, see figure 2 .
[0041] Get the plasmid of EPO-CTP fusion gene, use Not I and EcoR I to carry out double enzyme digestion ( image 3 ), recover the small fragments, and take the pcDNA eukaryotic expression vector at the same time, use Not I and EcoR I to carry out double enzyme digestion to recover the large fragments, connect the recovered two fragments with T4 ligase, transform them into E. coli DH5α, and screen for positive recomb...
Embodiment 2
[0042] Example 2 EPO-CTP fusion protein transfection of CHOK1 cell line
[0043] Transform the recombinant expression plasmid into a mammalian host cell line to express the EPO-CTP fusion protein. For high level stable expression, the preferred host cell line is CHOK1. The present invention adopts electrotransformation, 150 μg linearized DNA is added to 5 μl salmon sperm DNA, CHOK1 host cells are 5×106, after mixing, 180V electric shock is used twice, and the cell suspension after electrotransformation is spread into a plate with medium. When clonal clusters appeared in the plate, single clones were picked and expanded for culture. After 3 times of clonal screening, a relatively stable high-expressing cell line was obtained.
Embodiment 3
[0044] Example 3 Large-scale, high-density, carrier-attached perfusion culture of animal cells
[0045] The animal cell microcarrier-attached perfusion culture method mainly includes the inoculation of cells, the initial growth stage, changing the culture conditions, the subsequent growth stages and monitoring the culture conditions. The specific steps are as follows:
[0046] 1. Inoculation of cells.
[0047] A cell line expressing EPO-CTP was resuscitated in the cell bank, and passed three times in a T25 square bottle with DMEM medium plus 10% fetal bovine serum to adjust the cell state. When the cells are in the logarithmic growth phase, digest with trypsin, add serum to stop the digestion, and add 10% fetal bovine serum in DMEM medium for seed strand amplification, cell expansion according to T25-T75-T150 square flask- The order of the 2L spinner bottle was passed down step by step. Bioreactors with microcarriers were inoculated with spinner bottle digested cells. The c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com