A kind of sucrose isomerase mutant with improved stability and its construction method
A technology for sucrose isomerase and mutants, which is applied in the fields of genetic engineering and enzyme engineering, can solve the problem of low thermal stability of sucrose isomerase mutants, and achieve flexibility, unaffected enzyme activity, and thermal stability Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: Construction of the recombinant plasmid containing sucrose isomerase mutant
[0046] Specific steps are as follows:
[0047] (1) Construction of recombinant plasmid containing wild-type sucrose isomerase pdsi
[0048] The chemically synthesized wild-type sucrose isomerase pdsi whose nucleotide sequence is shown in SEQ ID NO.2 was ligated with the pXMJ19 vector after digestion with HindⅢ enzyme and EcoRI enzyme to prepare the recombinant vector pXMJ19-pdsi.
[0049] (2) Obtaining recombinant vectors containing mutants:
[0050] Using the whole plasmid PCR technology, the recombinant vector pXMJ19-pdsi prepared in step (1) was used as a template for site-directed mutagenesis to obtain recombinant plasmids pXMJ19-A100E, pXMJ19-I205M, pXMJ19-S563L, pXMJ19-N578M, and pXMJ19-G152P, pXMJ19-S328F, pXMJ19-E76R, pXMJ19-S563R, pXMJ19-V280L, pXMJ19-S499F, pXMJ19-V280L / S499F.
[0051] The primer sequences designed respectively are as follows:
[0052] A100E-F: GAGTAT...
Embodiment 2
[0074] Example 2: Construction of recombinant Escherichia coli engineering bacteria producing sucrose isomerase mutants and expression, isolation and purification of sucrose isomerase
[0075] Specific steps are as follows:
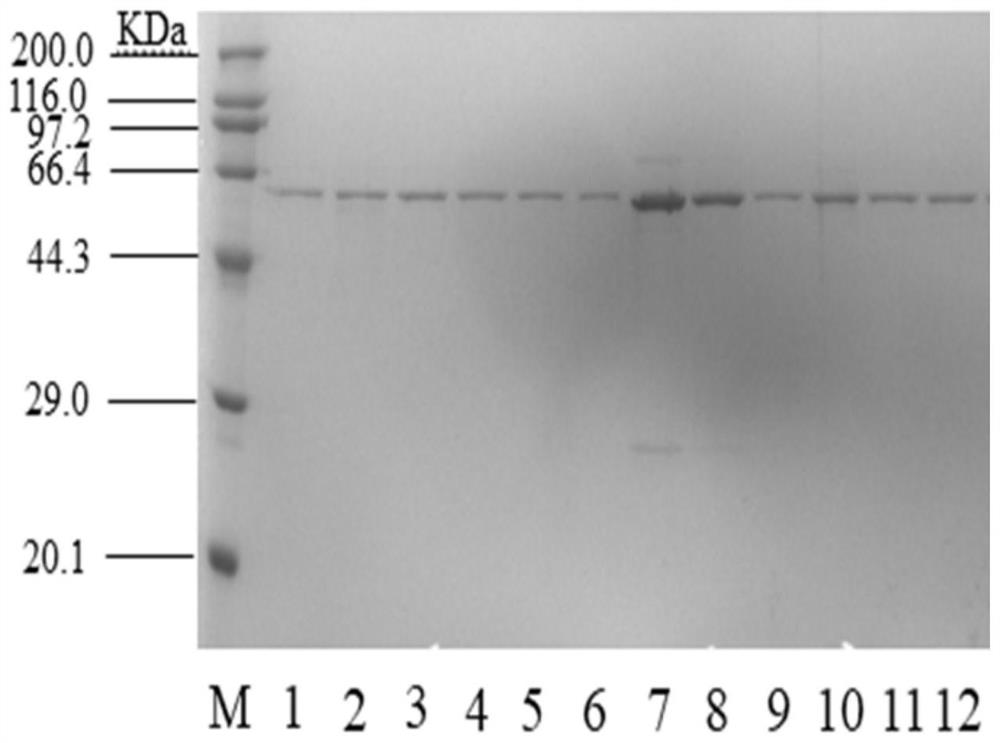
[0076] (1) The recombinant plasmids pXMJ19-pdsi, pXMJ19-A100E, pXMJ19-I205M, pXMJ19-S563L, pXMJ19-N578M, pXMJ19-G152P, pXMJ19-S328F, pXMJ19-E76R, pXMJ19-S563R, V280L, pXMJ19-S499F, pXMJ19-V280L / S499F were transformed into C.glutamium ATCC13032 competent cells by electroporation, and genetic engineering bacteria were prepared respectively: C.glutamium13032 / pXMJ19-pdsi, C.glutamium13032 / pXMJ19-A100E, C.glutamium13032 / pXMJ19-A100E, C. .glutamium13032 / pXMJ19-I205M,C.glutamium13032 / pXMJ19-S563L,C.glutamium13032 / pXMJ19-N578M,C.glutamium13032 / pXMJ19-G152P,C.glutamium13032 / pXMJ19-S328F,C.glutamium13032 / pXMJ19-E76R,C.glutamium13032 / pXMJ19-S563R, C. glutamium13032 / pXMJ19-V280L, C. glutamium13032 / pXMJ19-S499F, C. glutamium13032 / pXMJ19-V280L / S499F.
[0077] (2) In...
Embodiment 3
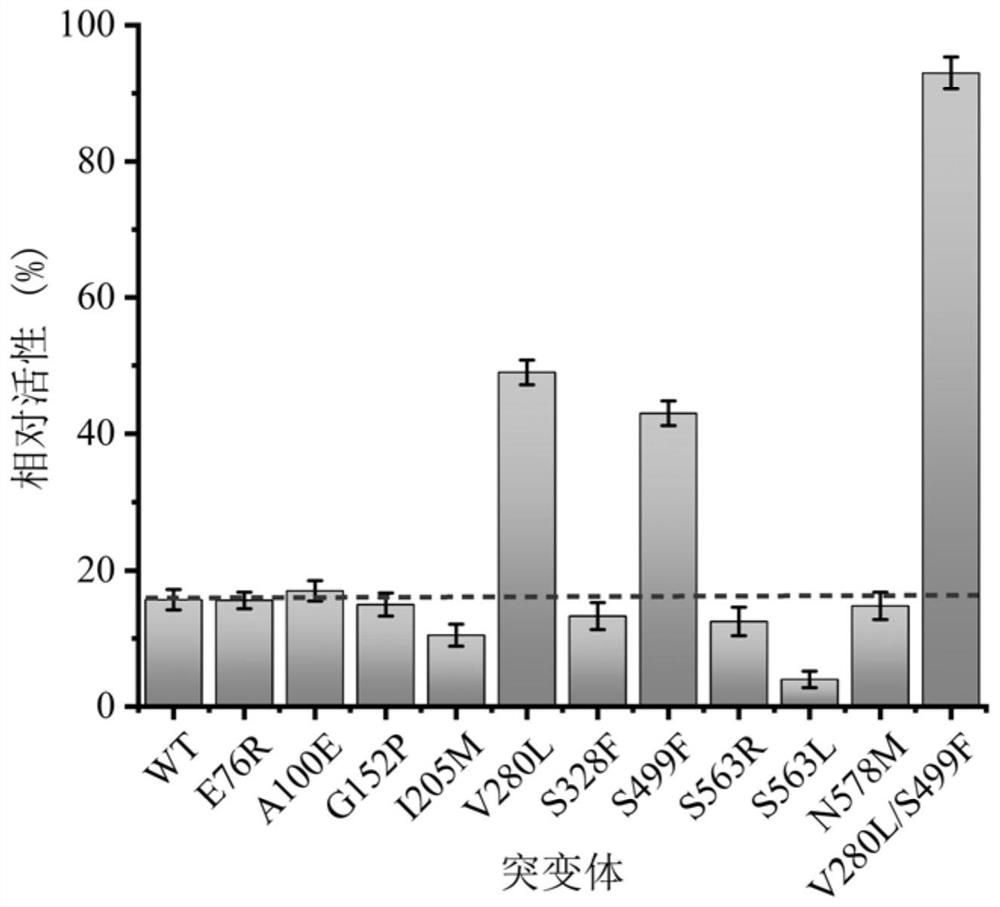
[0089]Embodiment 3: Enzymatic properties of sucrose isomerase mutant
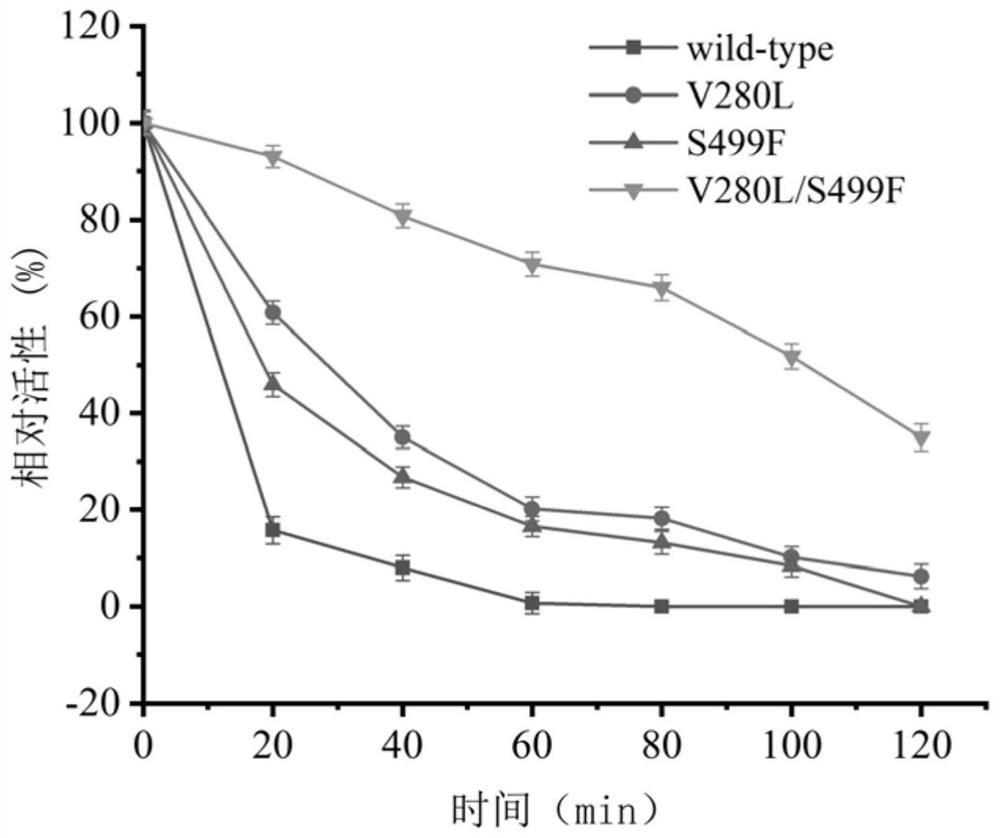
[0090] 1. Thermal stability
[0091] Take the pure enzyme solution containing wild-type pdsi, the pure enzyme solution containing V280L, the pure enzyme solution containing S499F, and the pure enzyme solution containing V280L / S499F prepared in step (2) of Example 2 and place them in a constant temperature water bath at 45°C , take samples at intervals, measure its residual enzyme activity according to the sucrose isomerase enzyme activity assay method, compare its thermal stability, and obtain the half-life results of wild-type pdsi and its mutants as shown in Table 2 and image 3 shown.
[0092] Table 2 Half-life of different sucrose isomerases
[0093]
[0094] 2. Optimal pH
[0095] The pure enzyme solution containing wild-type pdsi, the pure enzyme solution containing V280L, the pure enzyme solution containing S499F, and the pure enzyme solution containing V280L / S499F prepared in step (2) of Examp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com