Parathyroid hormone compositions and uses thereof
A parathyroid hormone and composition technology, applied in the field of oral delivery of polypeptides, can solve the problem that parathyroid hormone cannot be taken orally
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation of Teriparatide Enteric-Coated Capsules
[0053] Prepare coating solution:
[0054] 1.5 mg of Eudragit L-100, 3 mg of Talc and 1 mg of polyethylene glycol were dissolved in dichloromethane and isopropanol to obtain a coating solution.
[0055] To prepare enteric-coated capsules:
[0056] Mix teriparatide with Tween 80 (if there is Tween 20 in the composition, then mix teriparatide with Tween 20 and Tween 80), then add SBTI, mix, put into capsules, pack The coating liquid is sprayed onto the capsule surface to obtain teriparatide enteric-coated capsules. SBTI is soybean trypsin inhibitor.
Embodiment 2
[0058] Prepare the enteric-coated capsule of following composition according to embodiment 1
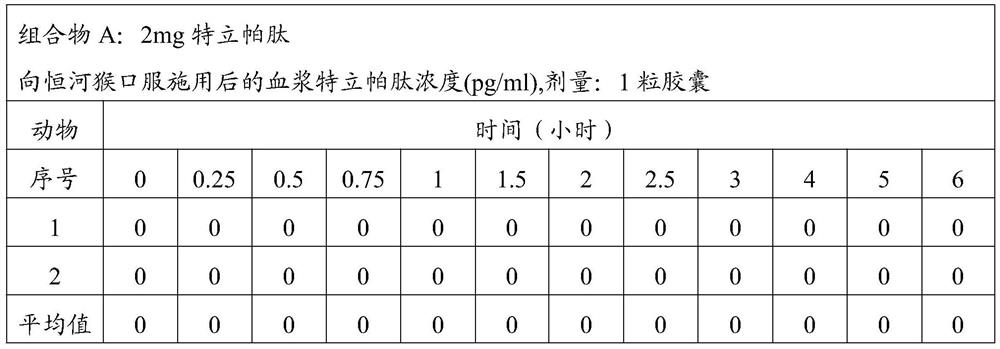
[0059] Composition A: 2mg teriparatide;
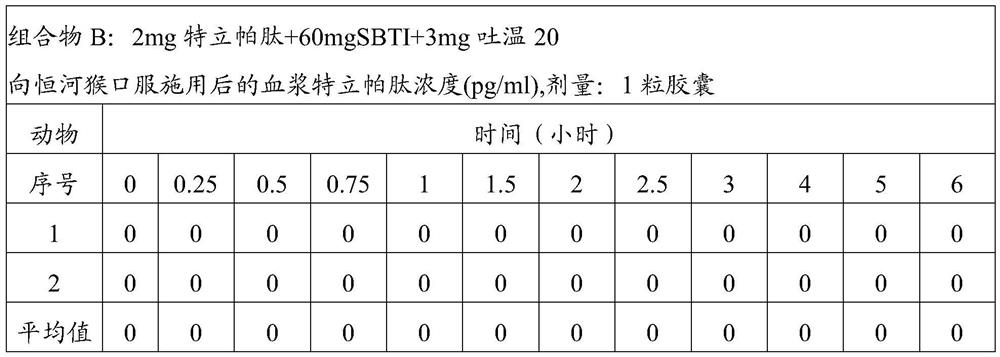
[0060] Composition B: 2 mg teriparatide + 60 mg SBTI + 3 mg Tween 20;
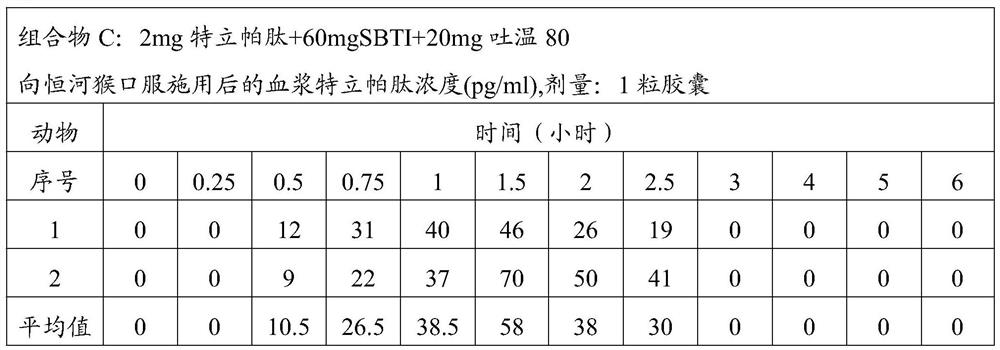
[0061] Composition C: 2mg teriparatide + 60mg SBTI + 20mg Tween 80;
[0062] Composition D: 2 mg teriparatide + 60 mg SBTI + 3 mg Tween 20 + 20 mg Tween 80;
[0063] Composition E: 2 mg teriparatide + 15 mg SBTI + 0.2 mg Tween 20;
[0064] Composition F: 2 mg teriparatide + 15 mg SBTI + 5 mg Tween 80;
[0065] Composition G: 2 mg Teriparatide + 15 mg SBTI + 0.2 mg Tween 20 + 5 mg Tween 80.
[0066] SBTI is soybean trypsin inhibitor. Those skilled in the art will understand that the SBTI can be Bowman-Birk trypsin inhibitor or Kunitz trypsin inhibitor or a combination of the two.
[0067] The above composition was administered to rhesus monkeys in the following manner. The monkeys were divided into groups, and each group of monkeys was administered the corresponding ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com