New echinococcosis antigen Murinoglobulin-2 protein
A protein and amino acid technology, applied in the fields of resistance to vector-borne diseases, peptide sources, instruments, etc., can solve problems such as insufficient specificity and/or sensitivity, difficulty in protein selection, and difficulty in isolating hydatid proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1, the acquisition of echinococcosis neoantigen Murinoglobulin-2 protein
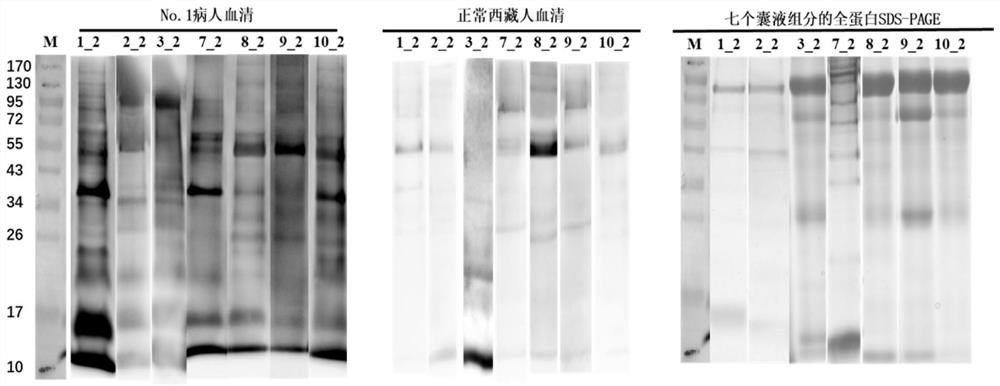
[0069] 1. The process of identifying more hydatid proteins from hydatid cysts isolated from hydatid patients after surgery
[0070] (1) Firstly, the hydatid cysts separated from 6 hydatid patients were divided into four parts according to their tissue structure to extract protein. Whether the cyst fluid is transparent or not, the 6 hydatid cysts were divided into a transparent group and an opaque group.
[0071] (2) The protein was extracted from the four components of each hydatid cyst, and the extracted protein was subjected to liquid enzymatic hydrolysis, and then identified by LC-MS / MS using a QE mass spectrometer.
[0072] (3) The raw data of QE off-machine was carried out using the maxquant protein identification software and the collection of human and hydatid protein databases (Unreviewed (TrEMBL) database downloaded from the Uniprot (https: / / www.uniprot.org / ) website) Hydatid p...
Embodiment 2
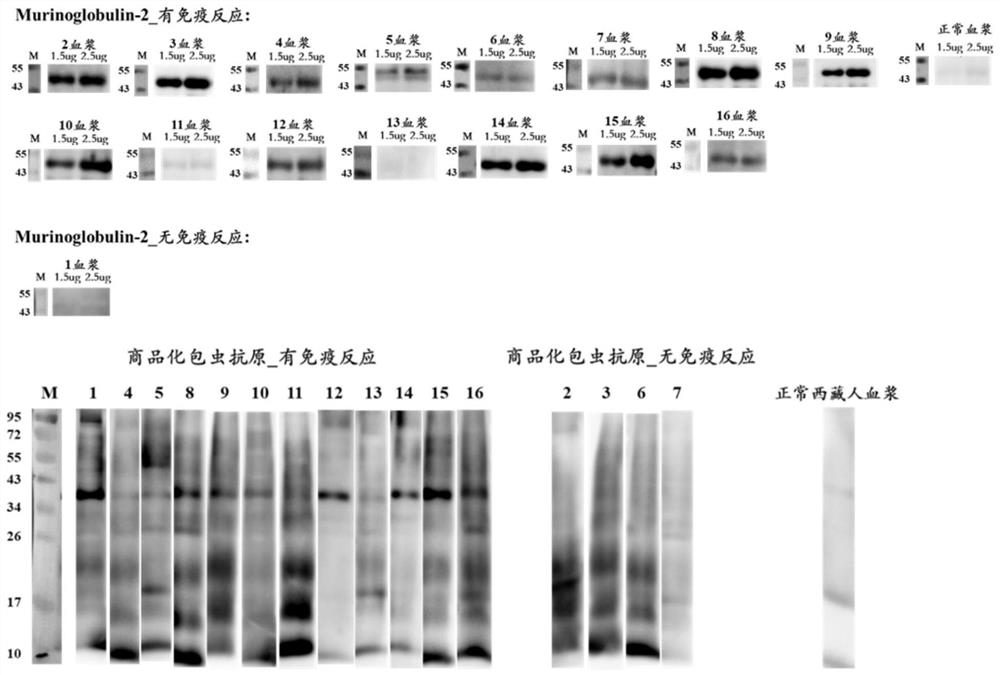
[0118] Example 2, ELISA Experimental Verification Case of Hydatid Recombinant Antigen Murinoglobulin-2 Recombinant Protein
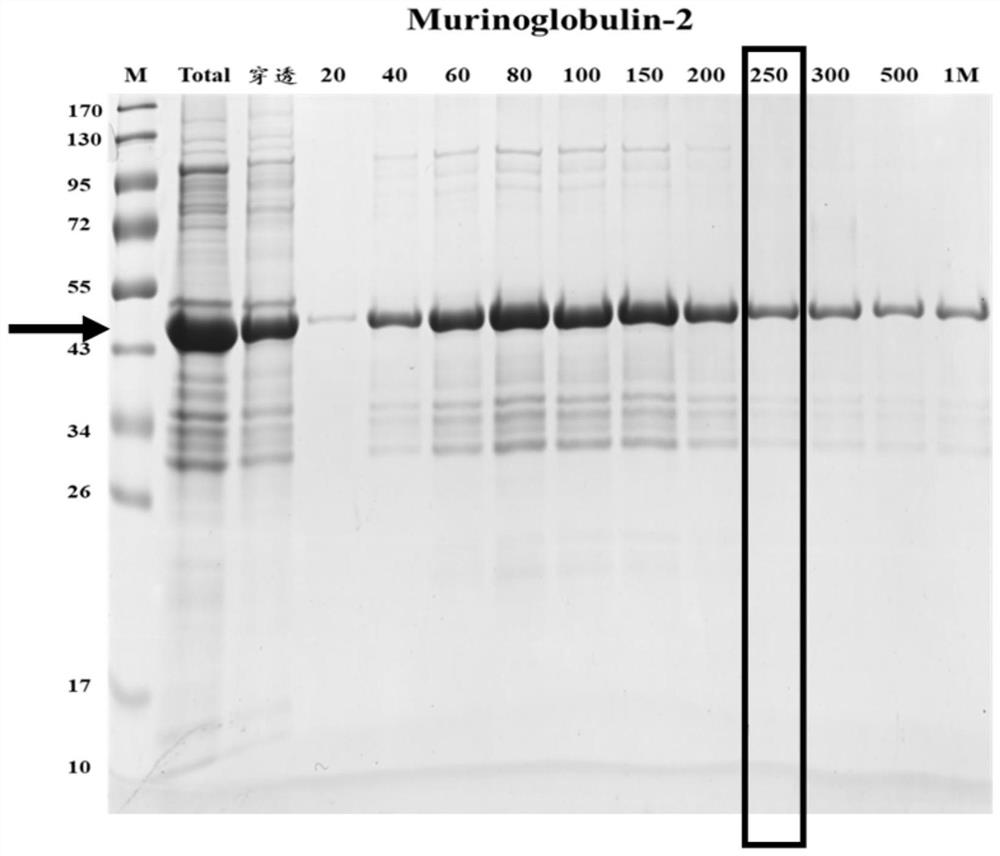
[0119] The detected objects were 14 postoperative echinococcosis patient plasma (clinically confirmed as positive) and 6 cases of normal Tibetan plasma (clinically confirmed as negative), which were carried out according to the standard operating procedures of indirect ELISA, and the results showed that Murinoglobulin-2 recombinant protein (purified in Example 1, i.e. figure 2 The target protein eluted when the middle imidazole concentration is 250mM) the positive detection rate is 86%, and the negative detection rate is 100%; Minuo Biotechnology Co., Ltd., product number: YM-VI08) when testing the same plasma, the positive detection rate was 86%, and the negative detection rate was 100%.
[0120] 1. ELISA experiment specific operation steps
[0121] (1) Antigen quantification: Each antigen was blown evenly with a pipette gun before coating, and a mic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com