Patents
Literature
65 results about "Echinococcoses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Echinococcus granulosusglutathione transferase gene and application thereof
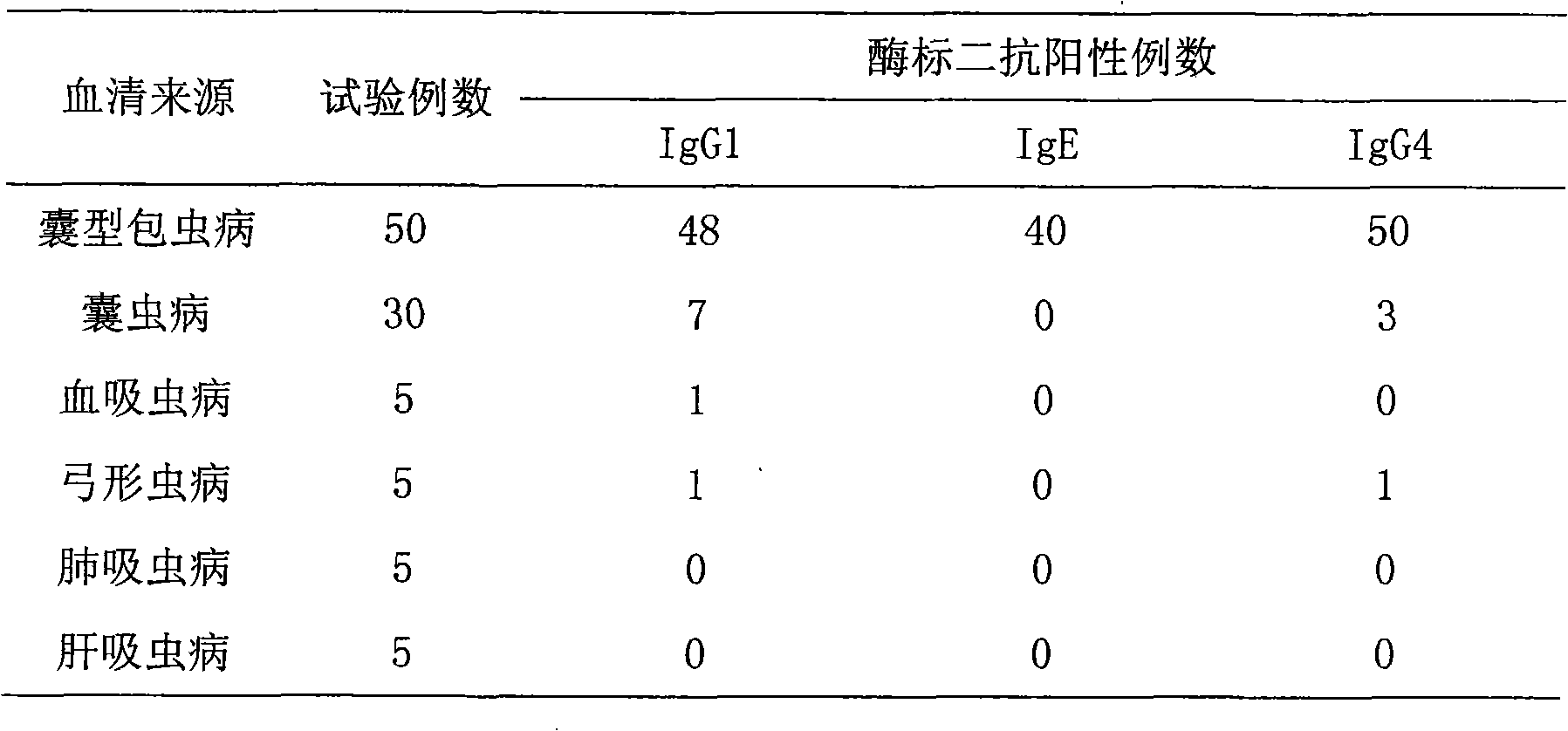
The invention belongs to the field of bioengineering and provides an Echinococcus granulosusglutathione transferase gene a base sequence of which is shown in SEQ ID NO: 1. The invention also provides a protein encoded by the Echinococcus granulosusglutathione transferase gene. The amino acid sequence of the protein encoded by the Echinococcus granulosusglutathione transferase gene is shown in SEQ ID NO: 2, or is the same as 1st-219th amino acid sequences as shown in SEQ ID NO: 3. The recombinant protein can be used for immunodiagnosis of cystic echinococcosis patients and can be used for coating a plate; and the serums of the patients having cystic echinococcosis, alveolitoid echinococcosis, cysticercosis and other parasite diseases and healthy people are detected by using an enzyme-linked immunosorbent assay (ELISA), and the obtained sensitivity and specificity are respectively 72.41% and 92.36%.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
PCR (Polymerase Chain Reaction) detection kit for cystic echinococcosis of dog
InactiveCN103374615AQuick and efficient detectionConvenient clinical diagnosis and treatmentMicrobiological testing/measurementAnimal fecesCystic echinococcosis
The invention discloses a PCR (Polymerase Chain Reaction) detection kit for cystic echinococcosis of a dog. According to the method, a pair of primers is designed according to a 12S rDNA (ribosome Deoxyribose Nucleic Acid) sequence conserved region of pathogene echinococcus granulosus of the cystic echinococcosis, a PCR reaction is carried out with the DNA contained in the wastes of an animal to be detected serving as a template, and then a comparison is carried out relative to an attached negative control and a positive control, in order to determine whether the detected animal infects the echinococcosis. The detection kit is simple and easy to use, and high in sensitivity and stability and can be used for monitoring the health condition of a pet dog.
Owner:WITHYOU BIOTECH
Immunochromatography test strip for detecting cystic echinococcosis and preparation method thereof
InactiveCN101881773AIncreased sensitivityImprove featuresMaterial analysisCelluloseEchinococcus antigen
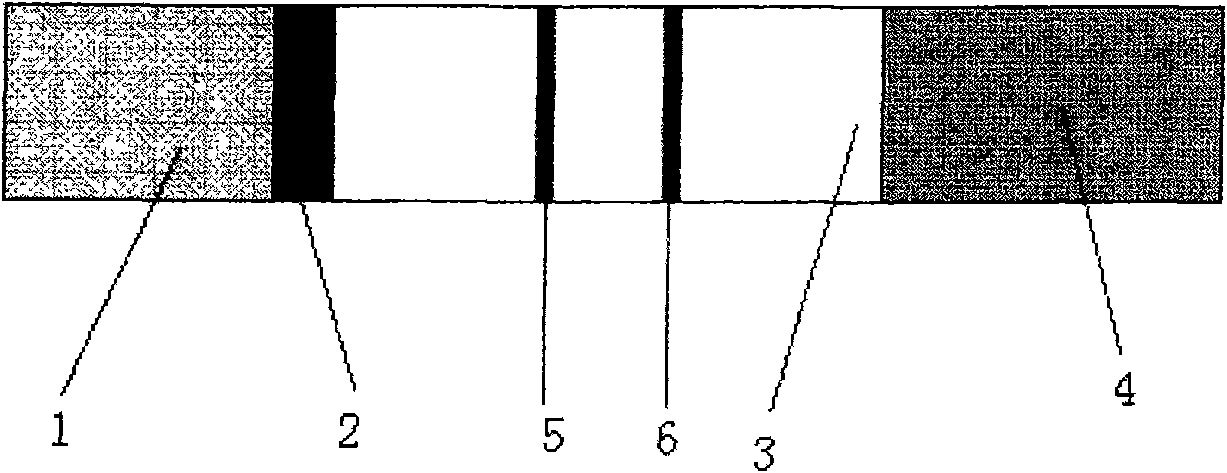
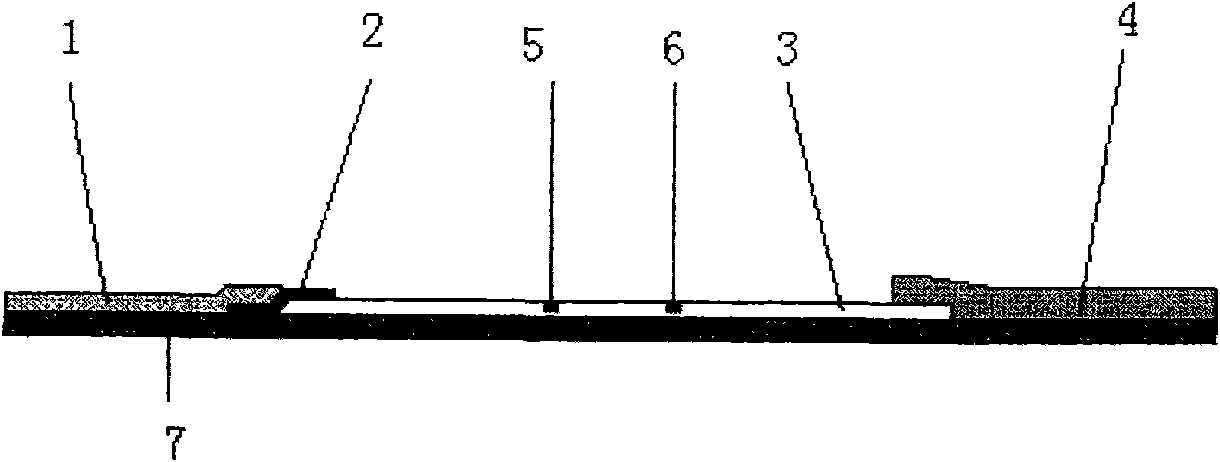
The invention discloses an immunochromatography test strip for detecting cystic echinococcosis and a preparation method thereof. The test strip comprises a hemofiltration membrane sample pad, a gold-marking pad, a cellulose membrane and a water absorption pad, wherein the gold-marking pad is tightly connected with the hemofiltration membrane sample pad and comprises an antibody labeling colloid gold probe of anti-echinococcus antigen advantage special antibody subtypes, the cellulose membrane is tightly connected with the gold-marking pad, the water absorption pad is tightly connected with the other end of the cellulose membrane, a detection line and a quality control line are arranged on the cellulose membrane, the detection line comprises crude antigens aiming at the cystic echinococcosis, and the quality control line comprises anti-antibodies capable of realizing special combination with the antibodies of the anti-echinococcus antigen advantage special antibody subtypes. The immunochromatography test strip of the invention has the advantages of simplicity, convenience, sensitivity, specificity and high speed, and is applicable to clinical and field use.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
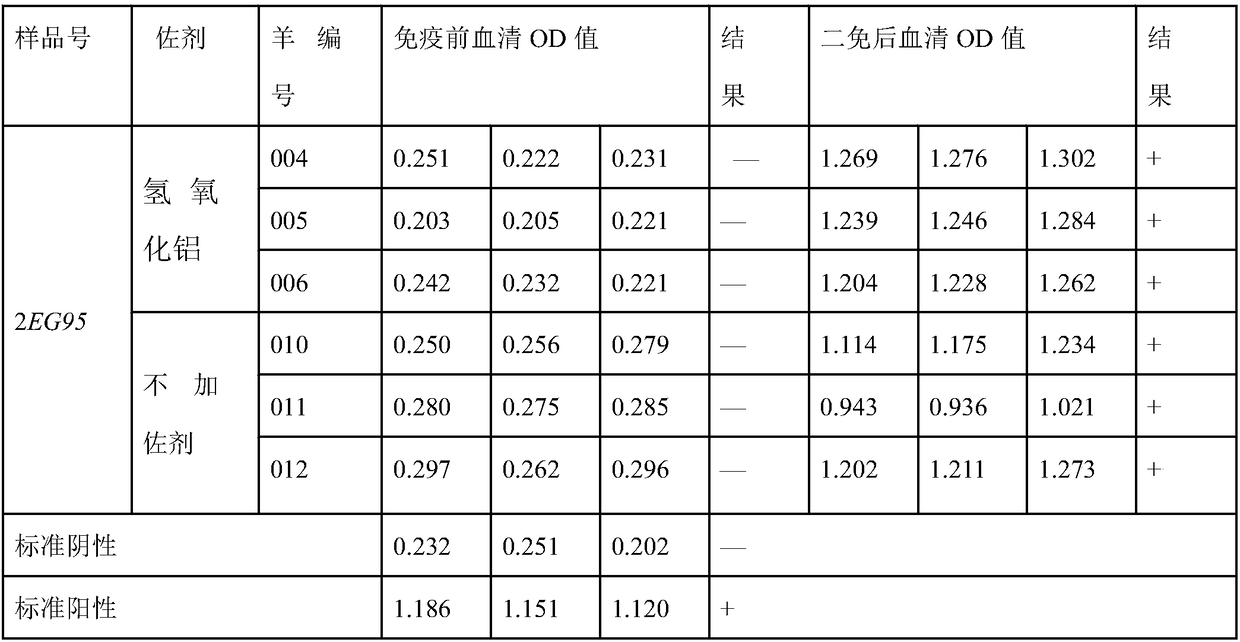
Sheep echinococcosis infection-resistant gene engineering subunit vaccine as well as preparation method and application thereof
ActiveCN108066755AImprove solubilityGood antigenicityAntiparasitic agentsAntibody medical ingredientsEscherichia coliAntigen
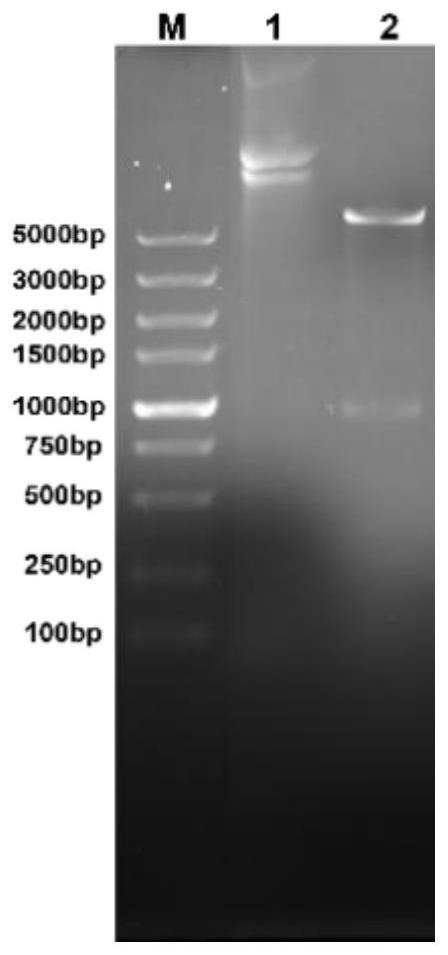
The invention discloses a preparation method of sheep echinococcosis infection-resistant gene engineering subunit vaccine. The preparation method comprises the following steps: S1, searching and downloading EG95 gene sequence as shown in SEQ ID NO.2 as well as EG95 amino acid sequence as shown in SEQ ID NO.3 of echinococcus granulosus from NCBI and performing amino acid sequence modification to obtain the modified EG95 amino acid sequence; S2, performing tandem expression on the gene sequences of a plurality of modified EG95 amino sequences through flexible linker to form recombinant multi-EG95 gene sequence; S3, cloning the recombinant multi-EG95 gene sequence to a pET28b plasmid vector, converting to Escherichia coli BL21 (DE3), and performing induction expression by a tag added fusion expression mode to obtain recombinant protein; and S4, performing protein purification on the recombinant protein to obtain the sheep echinococcosis infection-resistant gene engineering subunit vaccine. According to the preparation method, the production cost of echinotype antigen can be greatly reduced, the production process is greatly simplified and various advantages of safety, high efficiency,low cost and the like are achieved.
Owner:SA BIOTECH (SUZHOU) PTE LTD
Anti-echinococcosis antigen monoclonal antibody hybridoma cell strain, anti-echinococcosis antigen monoclonal antibody and application thereof
ActiveCN111269890AImmunoglobulins against animals/humansBiological material analysisBALB/cAdult worm
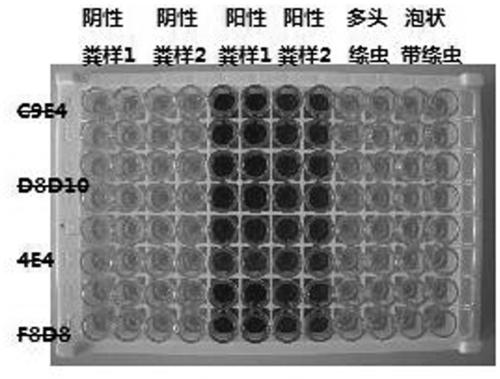
The invention relates to an anti-echinococcosis antigen monoclonal antibody hybridoma cell strain, an anti-echinococcosis antigen monoclonal antibody and application of the anti-echinococcosis antigenmonoclonal antibody. The anti-echinococcosis antigen monoclonal antibody hybridoma cell strain is classified and named as a hybridoma cell strain XJ10D8D10. The strain is preserved in the China Center for Type Culture Collection (CCTCC) on September 25th, 2017. The preservation address is Wuhan University, Wuhan, China, and the preservation number is CCTCC NO: C201789. According to the invention,an echinococcosis adult surface membrane antigen is extracted; a Balb / c mouse is immunized; splenic lymphocytes secreting anti-echinococcosis adult surface membrane antigen are obtained; the hybridoma cell strain secreting the anti-echinococcosis adult surface film antigen is obtained by fusing the splenic lymphocytes with SP2 / 0 cells, so that an echinococcosis-resistant adult surface film antigen antibody is obtained, and the echinococcus granulosus infection of dogs can be sensitively detected through fecal antigen detecting by using the antibody as a diagnostic reagent. And necessary conditions are provided for preparation of large-scale general survey detecting kits.
Owner:VETERINARY INST XINJINAG ACADEMY OF ANIMAL SCI CLINIC MEDICAL SCI RES CENT XINJIANG ACADEMY OF ANIMAL HUSBANDRY SCI
Detection card for quickly quantitative detection of echinococcosis antibody in serum
The invention discloses a detection card for quickly quantitative detection of echinococcosis antibody in serum. The detection card includes a detection card case and a test paper strip assembled therein. The test paper strip includes a plastic base board provided with pressure-sensitive adhesive, wherein a sample pad, a marker pad, a nitrocellulose membrane and water absorption paper are successively adhered to the base board. The marker pad is composed of a carrier base layer and a marker. The marker is a film formed by spray-coating the carrier base layer with lanthanum-series fluorescencedetection microspheres and lanthanum-series fluorescence quality control microspheres. The nitrocellulose membrane is coated with an echinococcosis recombinant antigen to form a detection line and rabbit-anti-chicken IgY antibody to form a quality control line. The marker includes the fluorescence detection microspheres labeled by the echinococcosis recombinant antigen and the fluorescence qualitycontrol microspheres labeled by the chicken IgY antibody. The detection card can achieve quick quantitative detection of the echinococcosis antibody in situ and has great practical and application values.
Owner:杭州微瑞科技有限公司
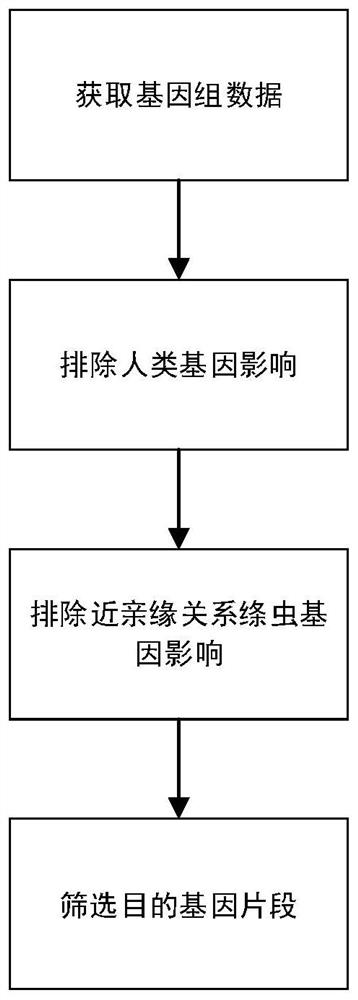
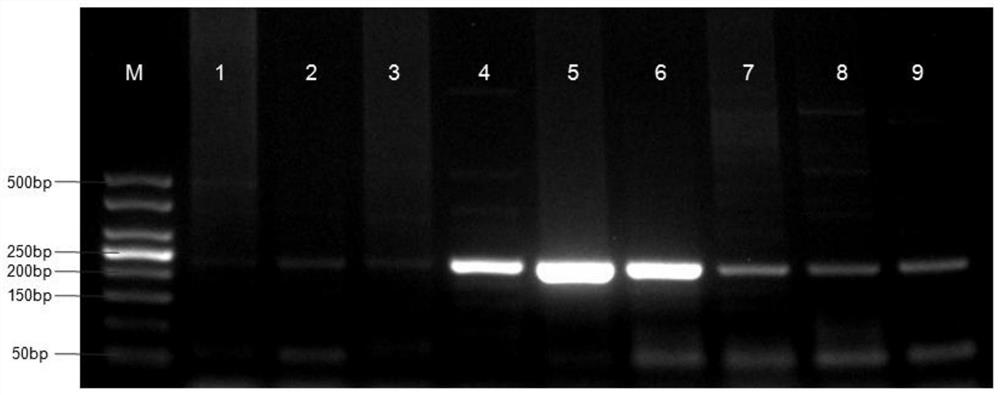
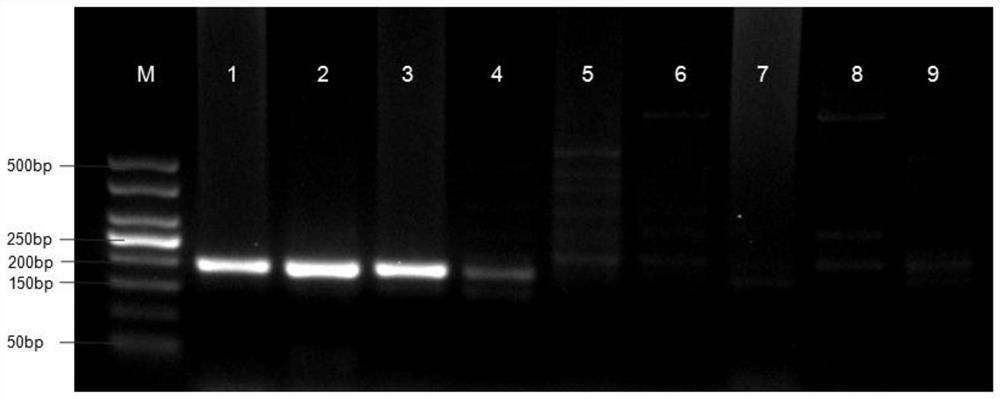
Liver echinococcus gene segment screening method, amplification primer and kit
PendingCN113355431AImprove accuracyImprove clinical outcomesMicrobiological testing/measurementSequence analysisHuman DNA sequencingEchinococcus multilocularis
The invention discloses a liver echinococcus gene segment screening method, an amplification primer and a kit.The screening method comprises the following steps: eliminating an influence of a human genome and a close genetic relationship tapeworm group genome from whole genomes of echinococcus granulosus and echinococcus multilocularis; and screening to obtain a third echinococcus granulosus gene segment, a third echinococcus multilocularis gene segment and a common gene segment, and designing three types of amplification primers by using three types of the gene segments respectively. A primer pair group for detecting echinococcosis of human tissues is obtained by further screening and a kit and a use method of the kit are provided based on the primer pair group. False positive results caused by human genes or close genetic relationship tapeworm genes existing in to-be-detected tissue DNA is avoided from the source, the to-be-detected DNA aiming at the primer during design is a human tissue sample, the false negative results in clinical detection are remarkably reduced, specific primers have higher accuracy and higher specificity, and clinical use effects of the primer pair and the kit are obviously enhanced.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Skeleton slow releasing implant for deworming from dogs and its preparation method
InactiveCN101007171AReduce the cost of prevention and treatmentReduce volumeOrganic active ingredientsPharmaceutical delivery mechanismDrugPlant agent
The invention discloses a domestic dog insect-repelling frame-typed slow-release plant agent and making method in the drug engineering technical domain, which consists of conventional echinococcosis-proof drug and tissue compatible material with weight rate at 1:0.5-1:30; the tissue compatible material is composed of biological decomposable typed material and non-biological decomposable typed material; the conventional echinococcosis-proof drug is weighed to blend with tissue compatible material according to certain proportion, which is dissolved in the organic solvent under indoor temperature or heating condition; the composition is grinded to add into heat mould, which is squeezed or shaped under indoor temperature after stripping mould.
Owner:SHANGHAI JIAO TONG UNIV
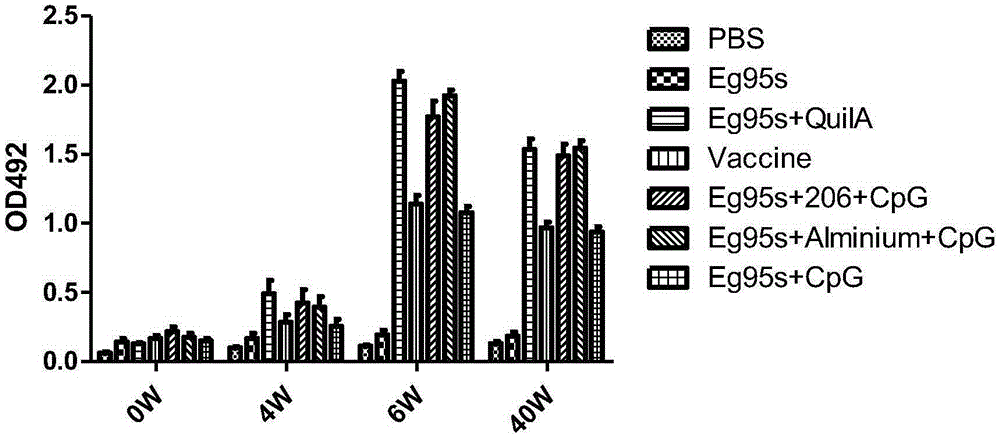
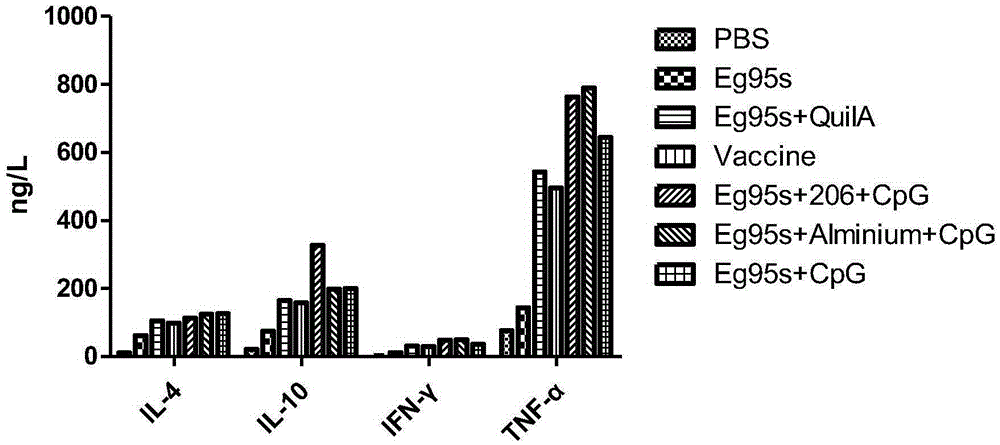
Novel echinococosis granulosis vaccine with CPG DNA (deoxyribonucleic acid) immune adjuvants
InactiveCN105267988AImprove immunityGood humoral immunityGenetic material ingredientsAntiparasitic agentsDna immunizationIntermediate host
The invention belongs to the technical field of vaccine development, and provides novel echinococosis granulosis vaccine for preventing intermediate hosts of echinococosis for human, livestock and the like. The novel echinococosis granulosis vaccine comprises soluble expression mixtures of Eg95 recombinant proteins, aluminum hydroxide and CpG immune adjuvants. The novel echinococosis granulosis vaccine has the advantage that excellent cellular immunity and humoral immunity of bodies can be effectively stimulated by the novel echinococosis granulosis vaccine.
Owner:BEIJING ZHONGNONG BIOLOGICAL ENG CO LTD
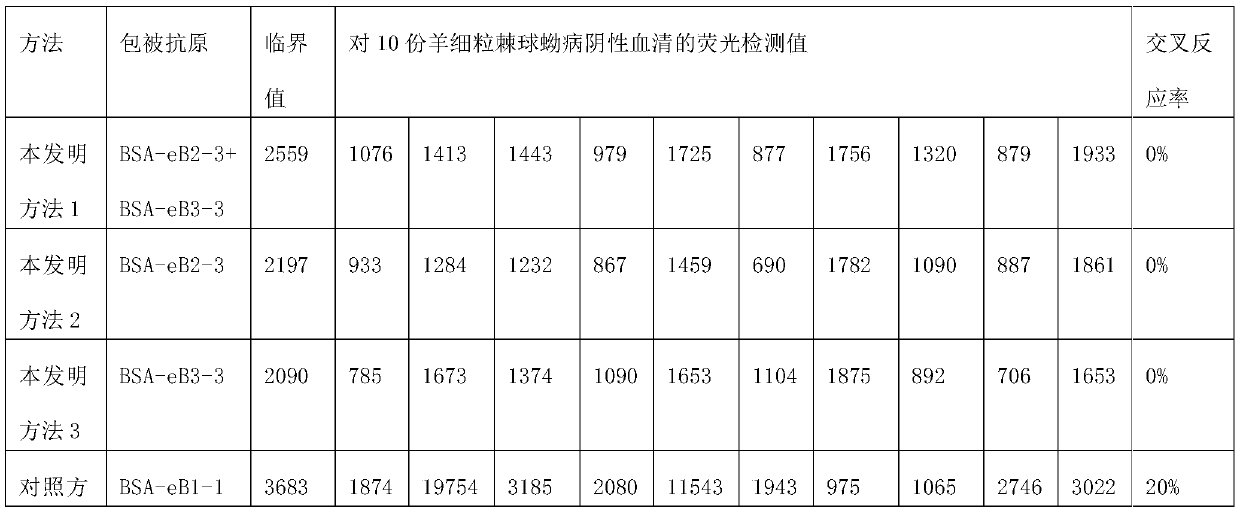
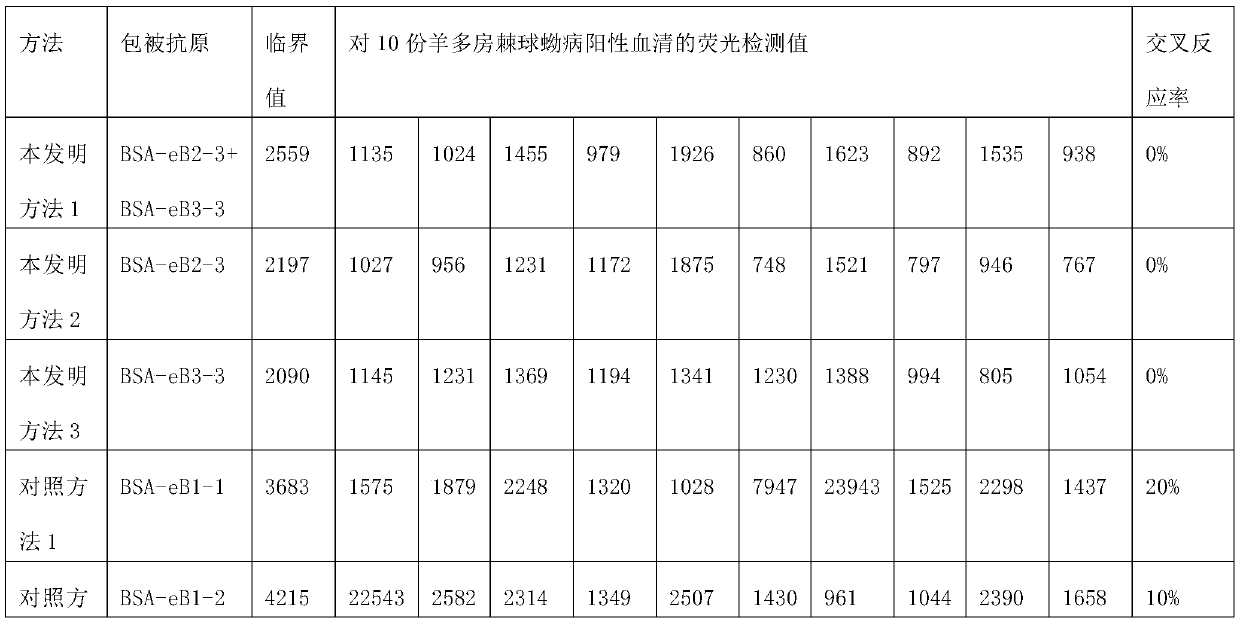
Cystic echinococcosis diagnostic kit and application thereof
ActiveCN110261615AStrong specificityIncreased sensitivityBiological material analysisBiological testingAntigenSerum ige
The invention discloses a cystic echinococcosis diagnostic kit and application thereof. The kit comprises a coating antigen composed of an eB2-3 conjugate and / or an eB3-3 conjugate; the eB2-3 conjugate represents a complete antigen obtained by coupling of eB2-3 with carrier protein; the eB3-3 conjugate represents a complete antigen obtained by coupling of eB2-3 with carrier protein; eB2-3 represents polypeptide shown by the sequence 1; and eVP1-2 represents polypeptide shown by the sequence 2. The kit is high in specificity, sensitivity and accuracy, simple and rapid in operation, and suitable for rapidly screening and detecting a lot of serum antibody infected by echinococcus granulosus by veterinary departments and entry-exit inspection and quarantine bureaus in all levels.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
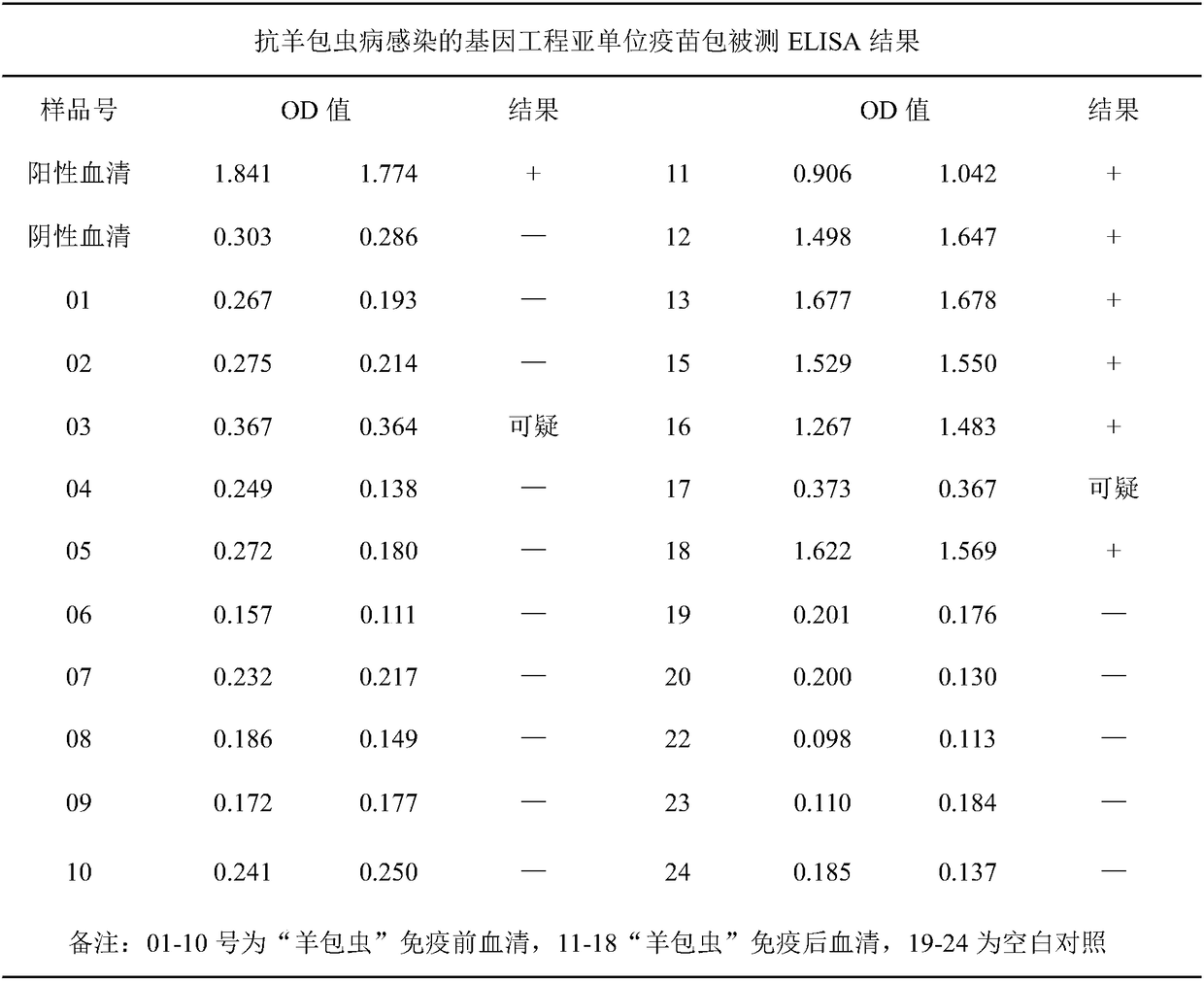
Sheep echinococcosis ELISA antibody detection kit and application thereof
PendingCN110954690AIncreased sensitivityImprove featuresBiological material analysisSheep serumEpidemiologic survey
The invention discloses a sheep echinococcosis ELISA antibody detection kit and a preparation method and application thereof. In the detection kit, an elisa plate is pre-coated with an echinococcus granulosus cocktail antigen; the echinococcus granulosus cocktail antigen is composed of an EPC1 recombinant antigen, an EgAgB1 recombinant antigen, an EgAgB2 recombinant antigen and an EgAgB4 recombinant antigen. The echinococcus granulosus cocktail antigen is composed of an EPC1 recombinant antigen, an EgAgB1 recombinant antigen, an EgAgB2 recombinant antigen and an EgAgB4 recombinant antigen. Thedetection kit provided by the invention can be used for rapidly detecting the echinococcosis antibody in the sheep serum, is simple to operate, short in consumed time, high in sensitivity and good inspecificity, and is convenient for simultaneously detecting a large number of samples. The sheep echinococcosis ELISA antibody detection kit provided by the invention has important application valuein echinococcosis antibody level detection and epidemiological investigation.
Owner:北京明日达科技发展有限责任公司
Echinococcus granulosus recombinant protein EgG1Y162-2 (4) and application thereof
PendingCN114874336APromote maturityImproving immunogenicityProtozoa antigen ingredientsAntibody mimetics/scaffoldsDendritic cellRecombinant vaccines
The invention discloses an echinococcus granulosus recombinant protein, which is characterized in that four EgG1Y162-2 protein fragments are connected in series through a linker sequence 'GSGGSG' to form the recombinant protein; experiments show that the recombinant protein vaccine can promote maturation of dendritic cells. The action principle and effect of the recombinant vaccine EgG1Y162-2 (4) are disclosed for the first time, and a foundation is laid for preparing vaccines or diagnostic kits for preventing and treating echinococcosis of people or livestock.
Owner:XINJIANG MEDICAL UNIV
Cystic echinococcosis cyst fluid degreasing antigen for detecting alveolar echinococcosis antibody, and preparation method thereof
ActiveCN103217524AStrong specificityImprove featuresMaterial analysisCysts fluidAlveolar echinococcosis
The invention relates to the technical field of human and animal alveolar echinococcosis (AE) antibody detections, specifically to cystic echinococcosis (CE) cyst fluid degreasing antigen for detecting AE antibody, and a preparation method thereof. According to the present invention, the CE cyst fluid degreasing antigen is used for detecting AE antibody by using an enzyme-linked immunosorbent test double antigen sandwich method so as to effectively increase quality of the detection reagent, and provide characteristics of strong specificity, high sensitivity, stable performance and wide uses; the CE cyst fluid degreasing antigen can be used for detecting human and animal AE antibodies so as to conveniently compare antibody contents of different animal varieties; and according to the immunological reaction principle, the CE cyst fluid degreasing antigen further can be used for preparing colloidal gold immunochromatography reagents and other rapid detection reagents for detecting AE antibodies so as to create favorable conditions for echinococcosis identification diagnosis, prevalence surveys and epidemic situation surveillance works.
Owner:新疆维吾尔自治区实验动物研究中心
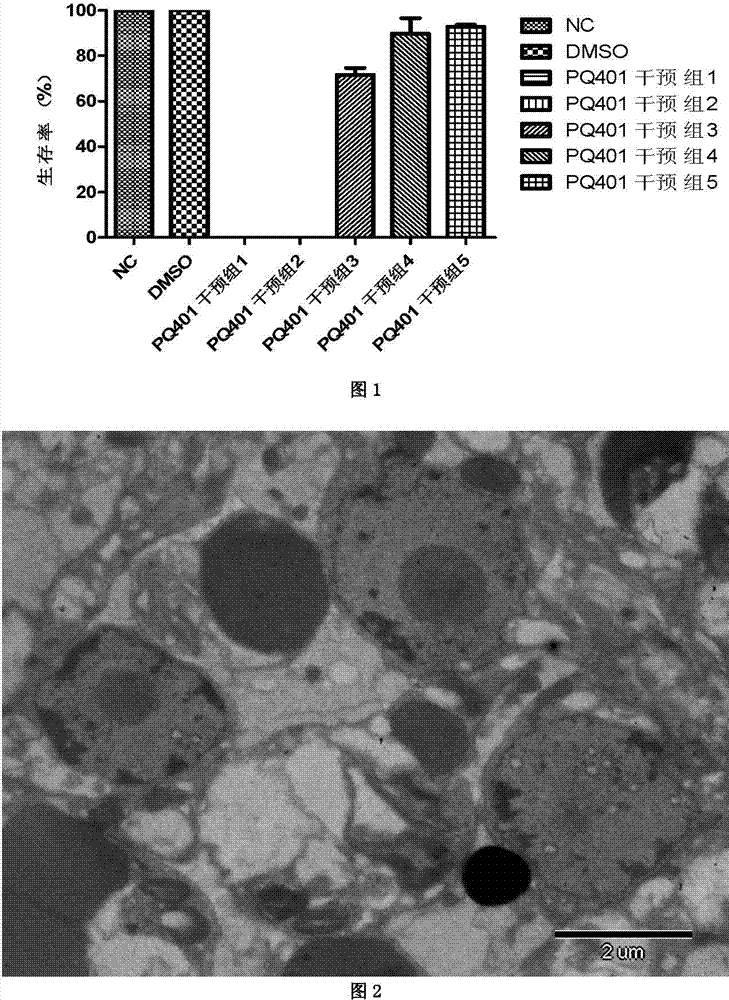
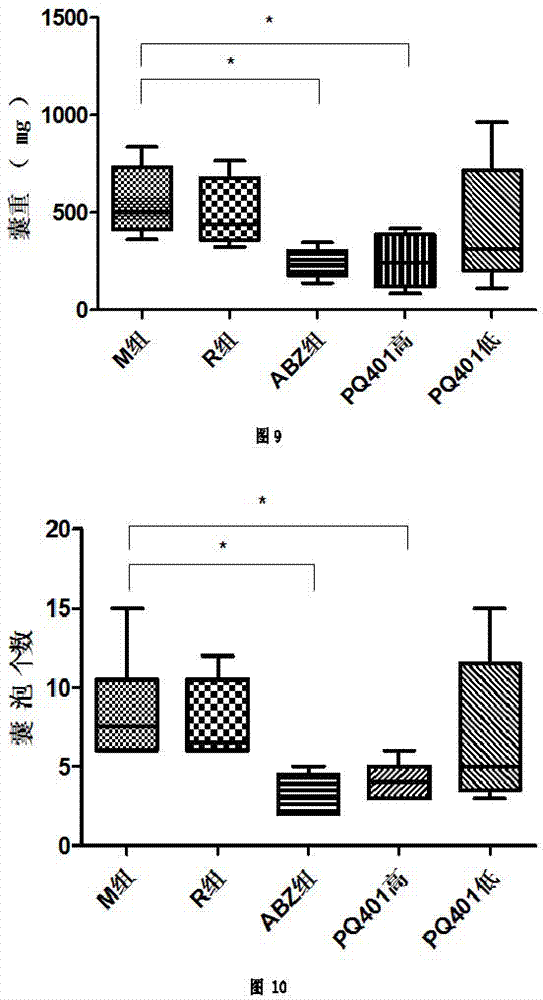
Application of insulin growth factor acceptor inhibitor PQ401 in as medicine for treating echinococcosis granulosa
The invention relates to the technical filed of an insulin growth factor acceptor inhibitor PQ401, and concretely relates to an application of the insulin growth factor acceptor inhibitor PQ401 as a medicine for treating echinococcosis granulosa. The insulin growth factor acceptor inhibitor PQ401 is a novel echinococcosis resistance medicine; in vivo and in vitro pharmacodynamic experiment data show that the PQ401 is an efficient echinococcosis resistance medicine molecule, can destroy the tissue structure of the Echinococcus granulosus vesicles and causes necrosis of a large amount of cells of the Echinococcus granulosus vesicles; in vivo pharmacodynamic experiment data show that the medicine has same echinococcosis granulose treatmetn effect with clinically commonly used albendazole; and the long term administration of albendazole by clinic echinococcosis granulosa patients causes medicine resistance, so the medicine can substitute albendazole to be used in the treatment of the echinococcosis granulosa.
Owner:THE FIRST TEACHING HOSPITAL OF XINJIANG MEDICAL UNIVERCITY
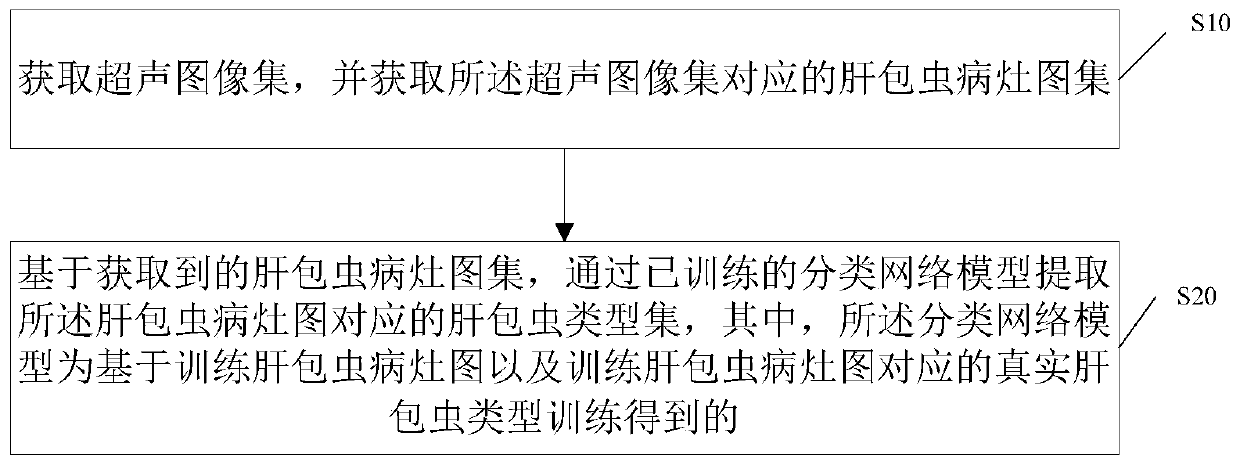
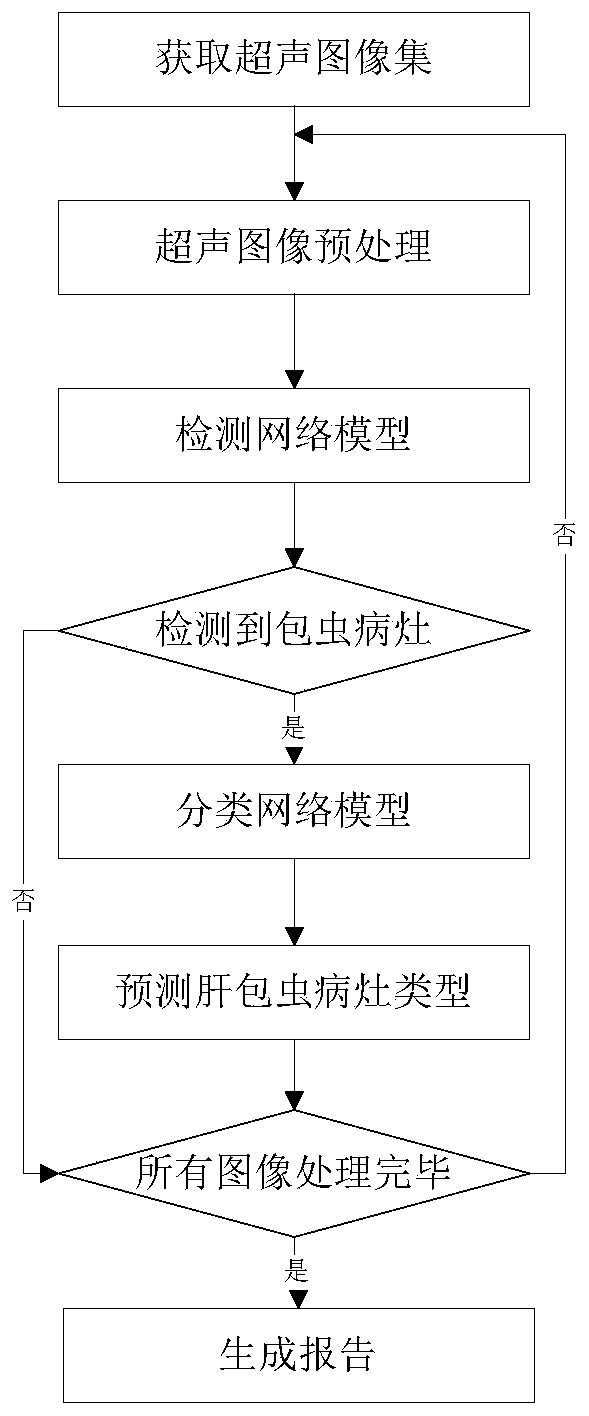
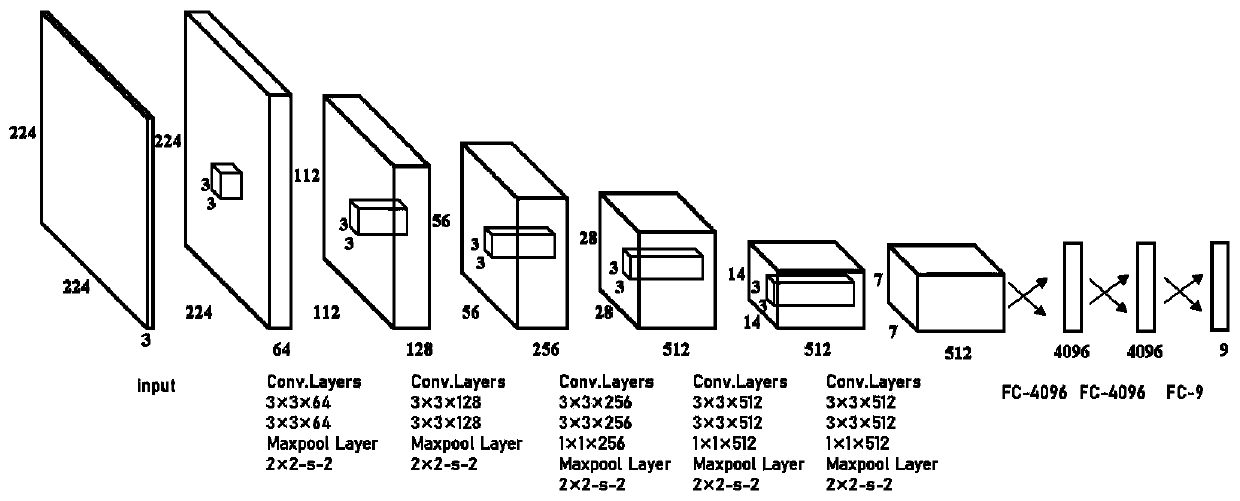
Hepatocystis recognition method based on ultrasonic image, storage medium and ultrasonic equipment
PendingCN111144506AImprove determination accuracyImprove accuracyImage enhancementImage analysisHepatic EchinococcosisRadiology
The invention discloses an echinococcosis identification method based on an ultrasonic image, a storage medium and ultrasonic equipment, and the identification method comprises the steps: obtaining anultrasonic image set, and obtaining an echinococcosis lesion atlas corresponding to the ultrasonic image set; based on the obtained liver echinococcosis lesion diagram set, extracting a liver echinococcosis type set corresponding to the liver echinococcosis lesion diagram through a trained classification network model to acquire the classification network model by training based on a training liver echinococcosis lesion diagram and a real liver echinococcosis type corresponding to the training liver echinococcosis lesion diagram. The hepatic echinococcosis lesion is determined through the ultrasonic image, and then the hepatic echinococcosis type is determined through the trained classification network model, so that the workload of doctors can be reduced, the accuracy of determining thehepatic echinococcosis type can be improved, and an accurate basis is provided for clinical treatment of hepatic echinococcosis.
Owner:MGI TECH CO LTD
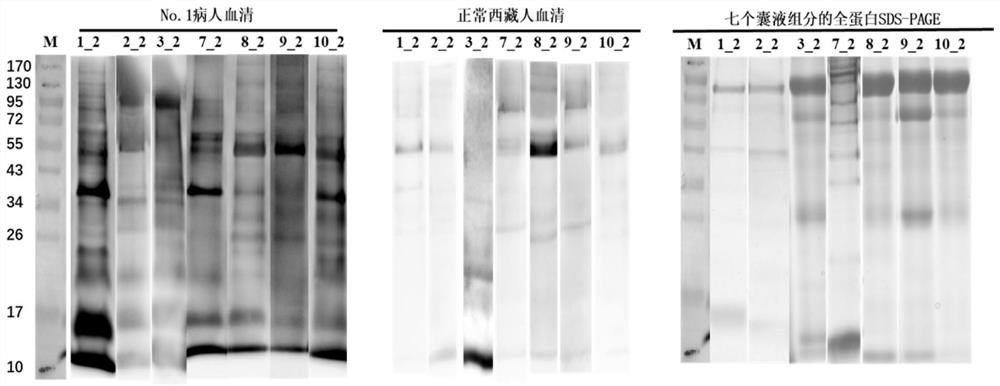
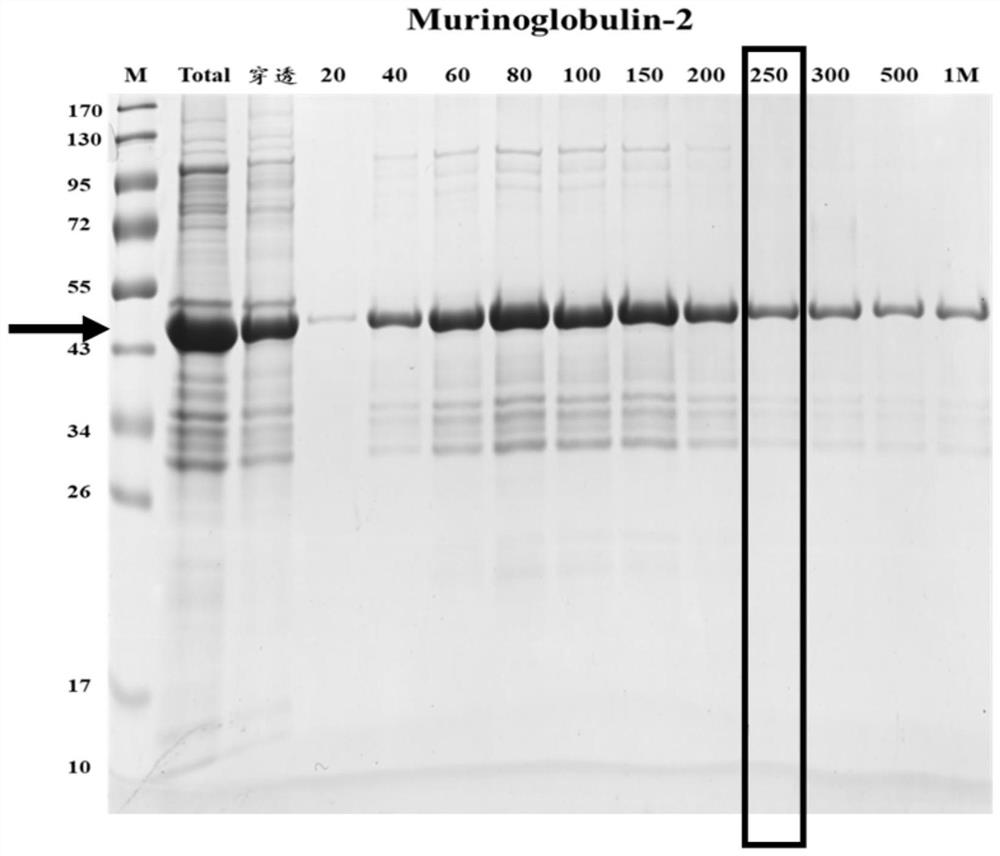
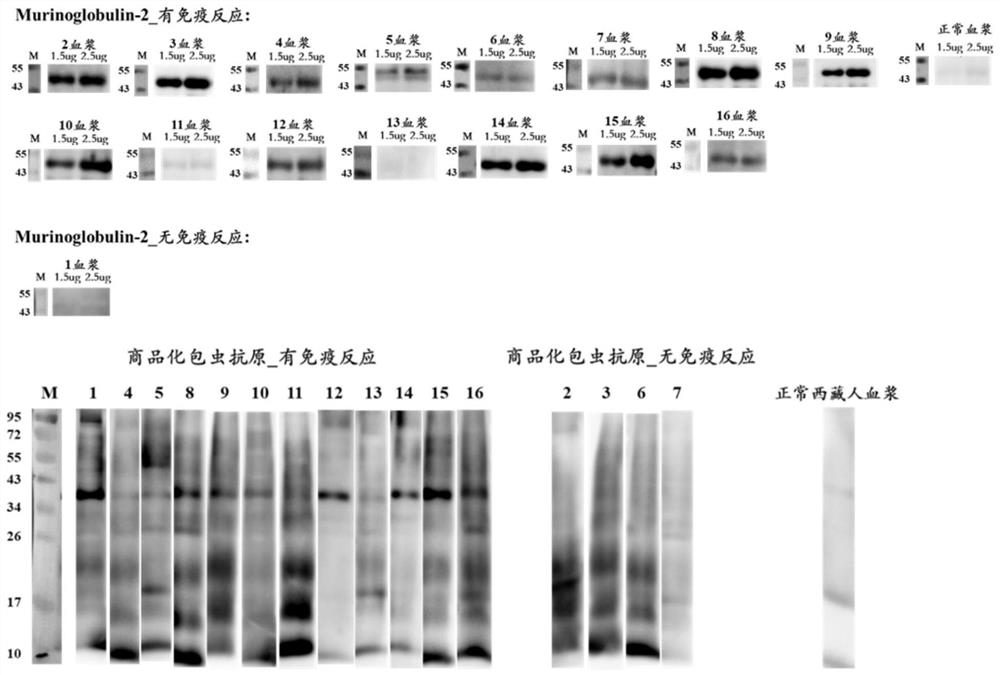
New echinococcosis antigen Murinoglobulin-2 protein
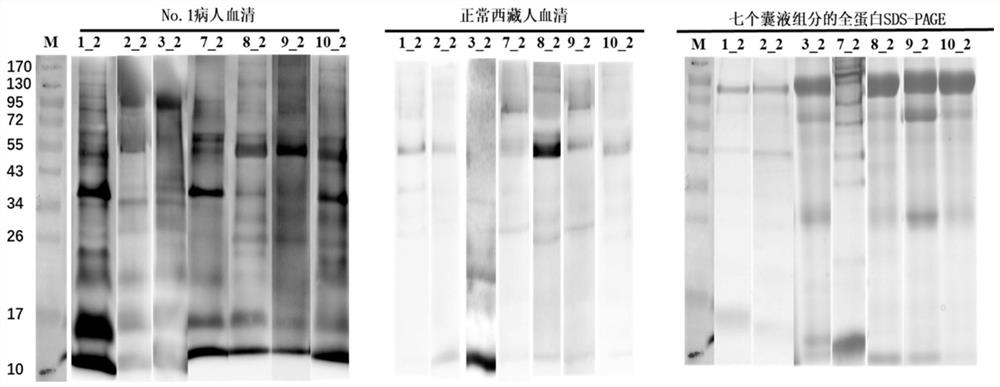
The invention discloses a novel echinococcosis antigen Murinoglobulin-2 protein. The amino acid sequence of the Murinoglobulin-2 protein provided by the invention contains all or part of the following sites: the 31st site to the 39th site, the 89th site to the 96th site, the 107th site to the 115th site, the 179th site to the 200th site, the 203rd site to the 216th site, the 225th site to the 242nd site, the 255th site to the 273rd site, the 282nd site to the 300th site, the 309th site to the 315th site, the 336th site to the 354th site and the 379th site to the 390th site of SEQ ID No.1. The Murinoglobulin-2 protein provided by the invention can be used for clinically detecting the human echinococcosis. The Murinoglobulin-2 protein is used for detecting plasma of 14 cases of postoperative echinococcosis people and plasma of 6 cases of normal Tibetan people, the positive detection rate is 86%, and the negative detection rate is 100%. According to the invention, a hydatid antigen library is expanded, and a certain contribution is made to diagnosis of human-derived echinococcosis.
Owner:BGI GENOMICS CO LTD
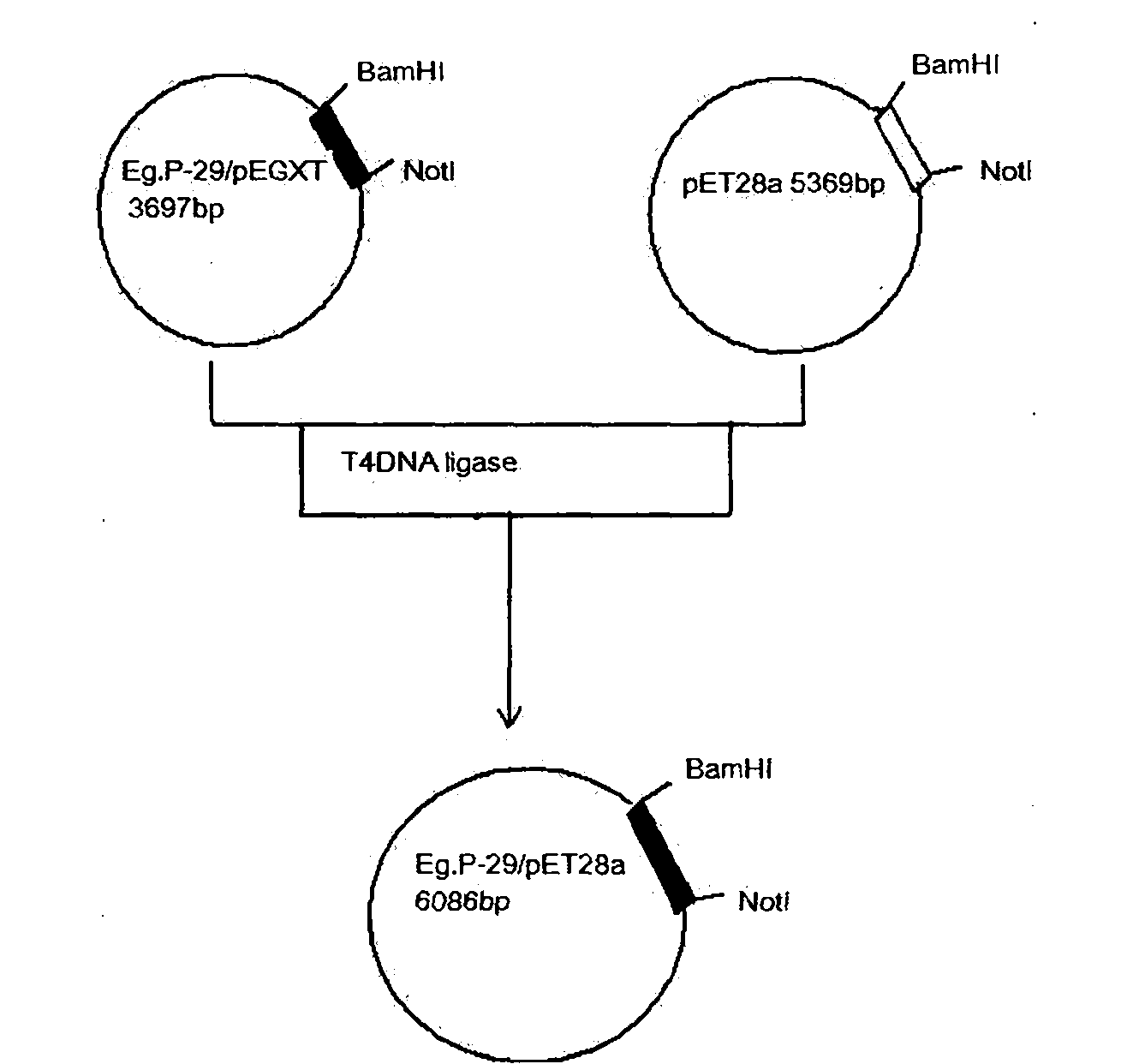
Echinococcosis granulosa gene engineering vaccine candidate P-29
InactiveCN101961487AGood antigenicityImproving immunogenicityAntiparasitic agentsAntibody medical ingredientsEscherichia coliAntigen
The invention provides an echinococcosis granulosa gene engineering vaccine. The sequence of the diagnostic antigen P-29 gene of the echinococcosis granulosa Uruguay strain is retrieved from the Genbank of www.ncbi.nlm.nih.Gov, the P-29 gene is amplified through RT-PCR, with the total RNA of the echinococcosis granulosa pathogens of echinococcosis patients in Ningxia region, China as the template, and the DNA sequence of the amplified gene is consistent with that of the gene of the Uruguay strain. The cloned P-29 gene and the expression vector pET-28a are recombined through the recombinant DNA technology to transform and construct the engineered escherichia coli strain. The P-29 recombinant protein is prepared and the obtained P-29 recombinant protein immune mice are attacked by the protoscolex pathogens of echinococcosis patients in Ningxia region. Through computation in the experiments, the protective immunity of EgP-29 is 96.6%. The P-29 recombinant protein is proved to have immunology effect of the vaccines.
Owner:赵巍 +2
Preparation and applications of echinococcus multilocularis resistant vesicle wall tissue (malpighian layer and cuticle layer) monoclonal antibody
ActiveCN103898064AImmunoglobulins against animals/humansMicroorganism based processesChemotherapy drugTreatment field
The invention discloses preparation and applications of echinococcus multilocularis resistant vesicle wall tissue (malpighian layer and cuticle layer) monoclonal antibody. The larva of echinococcus multilocularis parasitizes in human body and causes multilocular hydatidosis which is also known as echinococcus multilocularis disease. The larva usually parasitizes in the liver, and the disease is usually confused with tumors or other diseases. The invention relates to the scientific research, diagnosis, and treatment of echinococcus multilocularis disease, and provides a mouse-originated hybridoma cell strain. The monoclonal antibody secreted by the mouse-originated hybridoma cell strain can carry out specific binding with vesicle wall tissue (malpighian layer and cuticle layer) of echinococcus multilocularis, so the antibody can be used to carry out immunohistochemical specific staining on pathological tissues of the echinococcus multilocularis disease. The antibody titer can reach 1:105, and the antibody has a very strong sensitivity and specificity. The antibody can be used for the immunological pathological diagnosis of the echinococcus multilocularis disease. The monoclonal antibody can also be coupled with chemotherapy drugs or radionuclide to prepare target drugs and diagnosis reagents. The antibody can be applied to the scientific research related with echinococcus multilocularis too.
Owner:王昕 +2
A drug combination for preventing and treating animal echinococcosis and its application
ActiveCN107982264BReduce dosageAvoid drug resistanceAntiparasitic agentsHeterocyclic compound active ingredientsBiotechnologyWestern medicine
The invention relates to the technical field of composite formulae of traditional Chinese medicines and western medicines for livestock and in particular discloses a traditional Chinese medicine and western medicine composite preparation as well as a preparation method and an application thereof. The traditional Chinese medicine and western medicine composite preparation provided by the inventionis prepared from praziquantel, arecoline, cucurbitin, osthole and the like according to a certain ratio by using certain processes. The medicinal composition provided by the invention is simple and clear in method, easy to apply, remarkable in treatment effect due to the combination of traditional Chinese medicines and western medicines, and applicable to prevention and treatment on animal echinococcoses, and has relatively good economic benefits and social benefits.
Owner:WUHAN ACADEMY OF AGRI SCI +1
Method for detecting cystic echinococcosis
PendingCN113238056AAccurate detectionImprove accuracyBiological material analysisBiological testingSerodiagnosesSerum samples
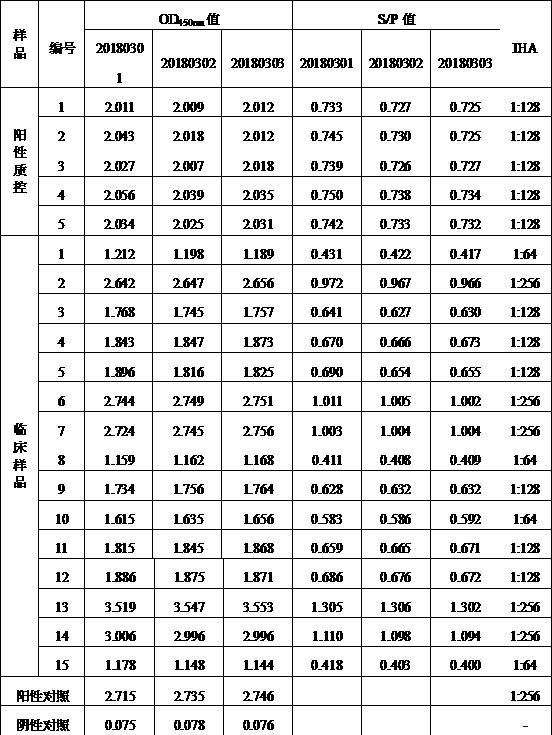
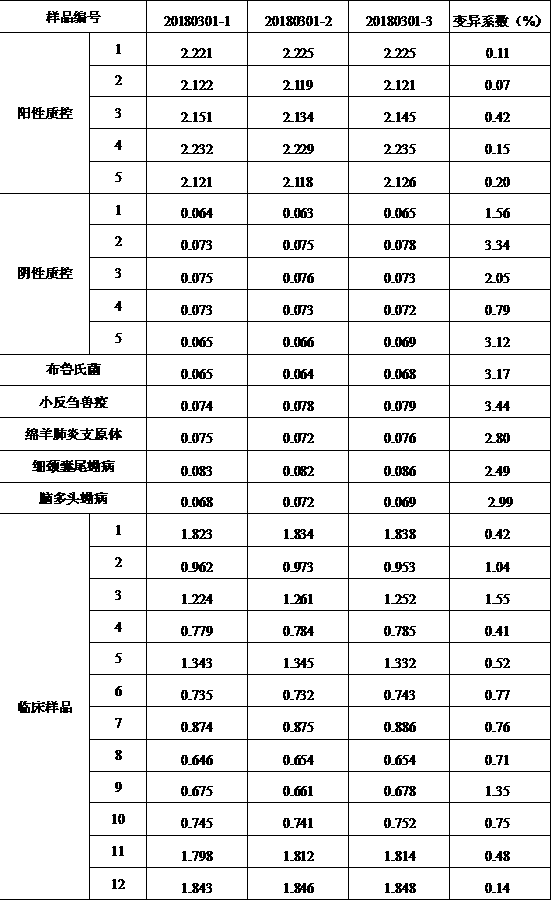
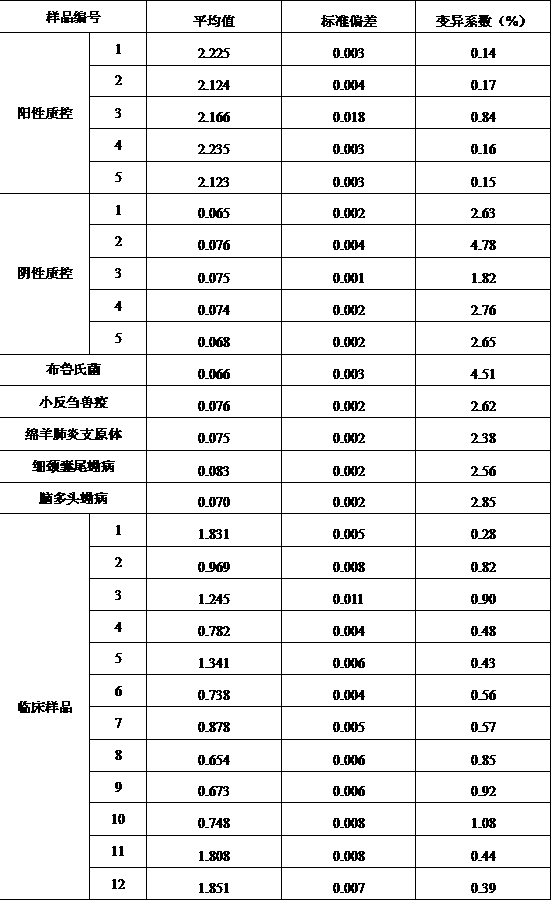
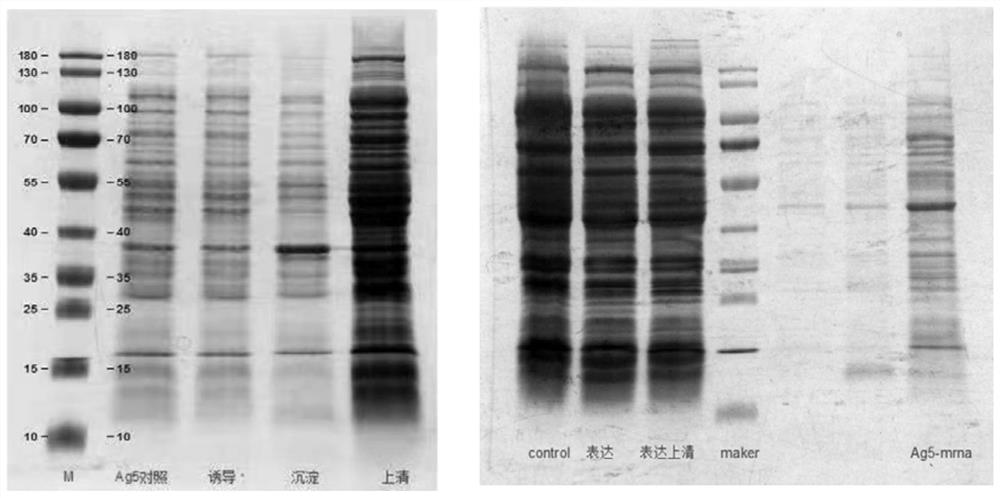
The embodiment of the invention provides a method for detecting cystic echinococcosis. The method comprises the following steps: coating an ELISA plate with an Ag5 antigen which is recombined and induced by a prokaryotic expression system and is purified in vitro; diluting serum of a subject in proportion, and adding the diluted serum and known negative and positive serum into the plate holes of the ELISAplate for reaction; and measuring the OD450 value of each plate hole, and calculating the sample value of each plate hole according to the publicity. Clinical serum sample detection shows that the detection specificity and sensitivity are higher than those of a commercial kit, and false positive results are effectively eliminated. The indirect ELISA method established in the invention provides a more accurate and effective detection means for serological diagnosis of echinococcosis.
Owner:中国人民解放军陆军第九五八医院
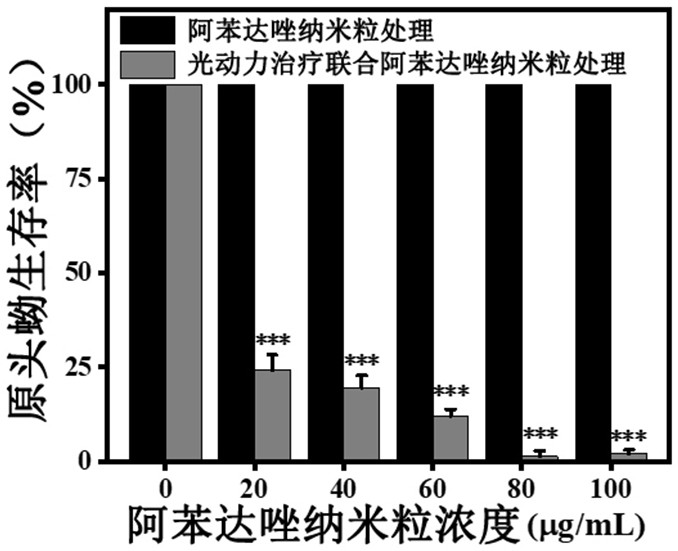
Composition of photodynamic combination drug as well as preparation method and application thereof
ActiveCN111744014ASmall toxicityReduce resistancePowder deliveryOrganic active ingredientsCystTetrandrine
The invention relates to the technical field of preparation of medicines for treating echinococcosis, and relates to a composition of a photodynamic combination drug and a preparation method and an application thereof. The composition comprises a photosensitizer or / and a chemical drug; wherein the photosensitizer is one or more of chlorin e6, viltipofen, indocyanine green, porphin sodium, 5-aminolevulinic acid, temopofen and talapofen, and the chemical drug is one or more of albendazole, albendazole sulfoxide, mebendazole, flubendazole, oxfendazole, artesunate, peganum harmala, tetrandrine andsophora japonica. According to the invention, through photodynamic therapy, the permeability of the hydatid and the hydatid cyst wall is increased; according to the invention, the target accumulationof chemotherapeutic drugs in the echinococcosis and the cyst wall is realized, the synergistic treatment effect is achieved, the resistance of the echinococcosis and the cyst to external drugs is reduced, the curative effect of the anti-echinococcosis drugs is improved, the administration dosage and the toxic and side effects of the drugs can be greatly reduced, and the application prospect in the preparation of echinococcosis treatment drugs is excellent.
Owner:XINJIANG MEDICAL UNIV +1
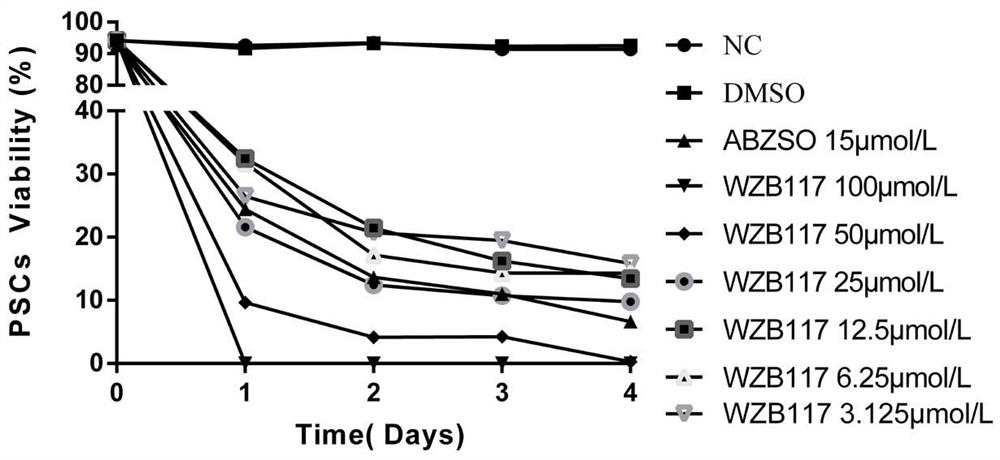
Application of glucose transporter 1 inhibitor WZB117 to preparation of cystic echinococcosis treating drug
PendingCN111888348AGood treatment effectGood synergistic effectOrganic active ingredientsAntiparasitic agentsMetaboliteTherapeutic effect
The invention relates to the technical field of echinococcosis drugs, in particular to an application of a glucose transporter 1 inhibitor WZB117 to preparation of a cystic echinococcosis treating drug. The invention discloses the application of the WZB117 to preparation of the cystic echinococcosis treating drug for the first time. Pharmacodynamic experiment data in vivo and in vitro show that the WZB117 is an efficient echinococcosis-resistant drug molecule, and particularly has a remarkable treatment effect on cystic echinococcosis, and the effect is superior to that of albendazole sulfoxide which is an albendazole metabolite; the combined use of the WZB117 and the albendazole sulfoxide has a better synergistic treatment effect, and the effect is remarkably superior to that of independent administration of the WZB117 and independent administration of the albendazole sulfoxide; and meanwhile, the dosage of the combined use of the WZB117 and the albendazole sulfoxide is reduced by 1 / 3-1 / 2 compared with the dosage of the independent administration of the albendazole sulfoxide, so that the dosage and the drug resistance are greatly reduced, a good treatment effect is achieved, and the WZB117 can be used as an effective substitute for existing albendazole.
Owner:THE FIRST TEACHING HOSPITAL OF XINJIANG MEDICAL UNIVERCITY
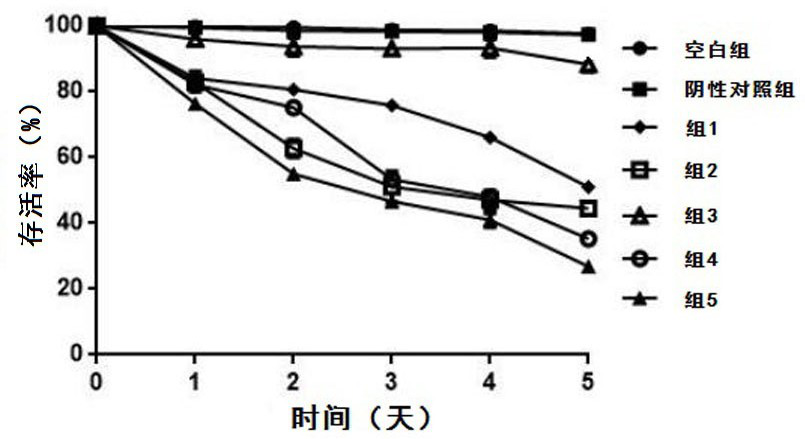
Composition combining DNA damage causing compounds with DNA damage repair inhibitors, and preparation method and application of composition
InactiveCN111714638AGood treatment effectOrganic active ingredientsCyclic peptide ingredientsRAD51Chemical compound
The invention relates to the technical field of preparation of medicine for treating echinococcosis, in particular to a composition combining DNA damage causing compounds with DNA damage repair inhibitors, and a preparation method and application of the composition. The composition comprises the DNA damage causing compounds and the DNA damage repair inhibitors. The DNA damage causing compounds areone or more kind of materials of harmine, harmine derivatives, adriamycin, dactinomycin, daunorubicin, etoposide and teniposide; and the DNA damage repair inhibitors are one or more kind of materialsof an RAD51 inhibitor and a BRCA1 inhibitor. Through combined use of the DNA damage causing compounds and the DNA damage repair inhibitors, the composition provided by the invention is used for treating the echinococcosis; the effect of realizing the damage to worm body DNA by the DNA damage causing compounds and realizing the inhibition on the DNA damage repair by the DNA damage repair inhibitors at the same time can be achieved; the worm body DNA damage repair process is completely blocked; finally, the worm body apoptosis is caused; the anti-echinococcosis effect is achieved; and the anti-echinococcosis treatment effect is improved.
Owner:THE FIRST TEACHING HOSPITAL OF XINJIANG MEDICAL UNIVERCITY +1
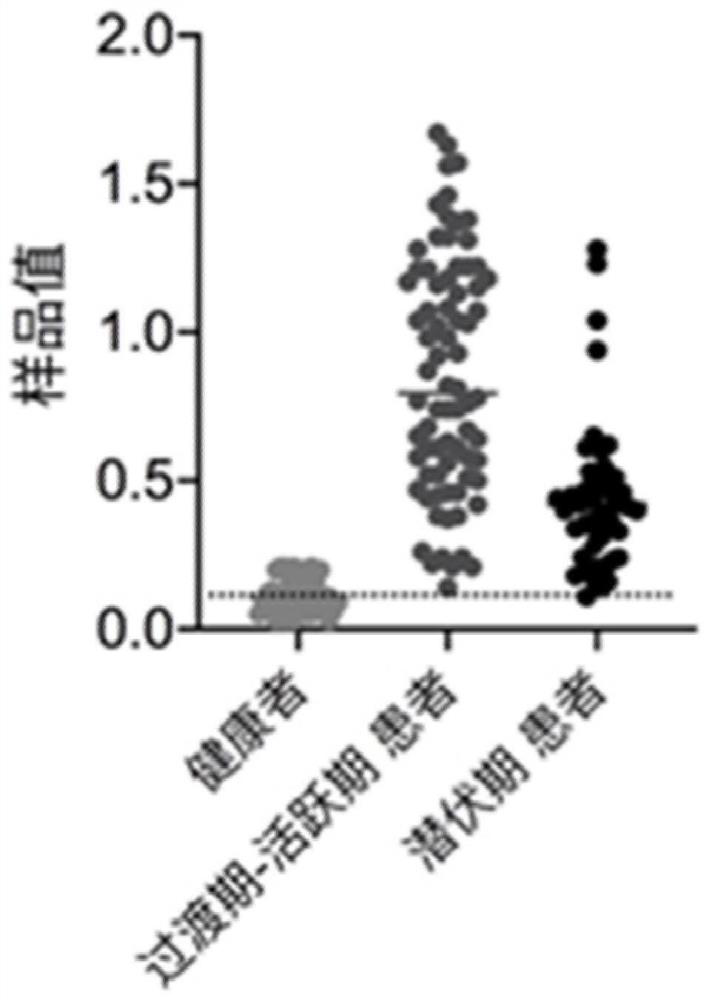
Early diagnosis and treatment biomarker for alveolar echinococcosis and application thereof
The invention discloses an early diagnosis and treatment biomarker for alveolar echinococcosis and application thereof. The invention protects application of a substance for detecting target proteinsin preparation of a product for diagnosis or auxiliary diagnosis of alveolar echinococcosis. The target proteins include (a1) or (a2) or (a3): the (a1) comprises ALDH1A1 protein, TAGLN2 protein and FLN alpha protein; the (a2) comprises any two of ALDH1A1 protein, TAGLN2 protein and FLN alpha protein; and the (a3) comprises ALDH1A1 protein or TAGLN2 protein or FLN alpha protein. The inventors of the invention use proteome correlation analysis of liver and plasma to detect potential candidate biomarkers in AE patients. Results show that ALDH1A1, TAGLN2 and FLN alpha may be early prediction indexes of the AE patients and can be used as candidate diagnosis indexes of early AE patients.
Owner:QINGHAI UNIV AFFILIATED HOSPITAL +1
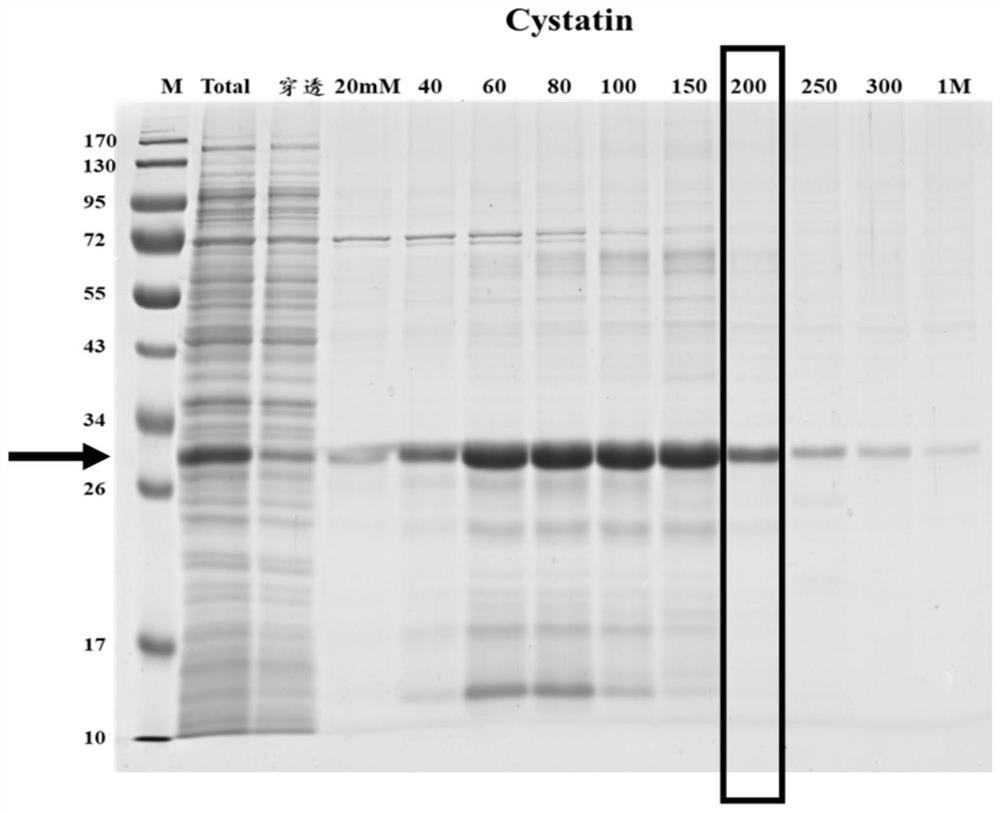
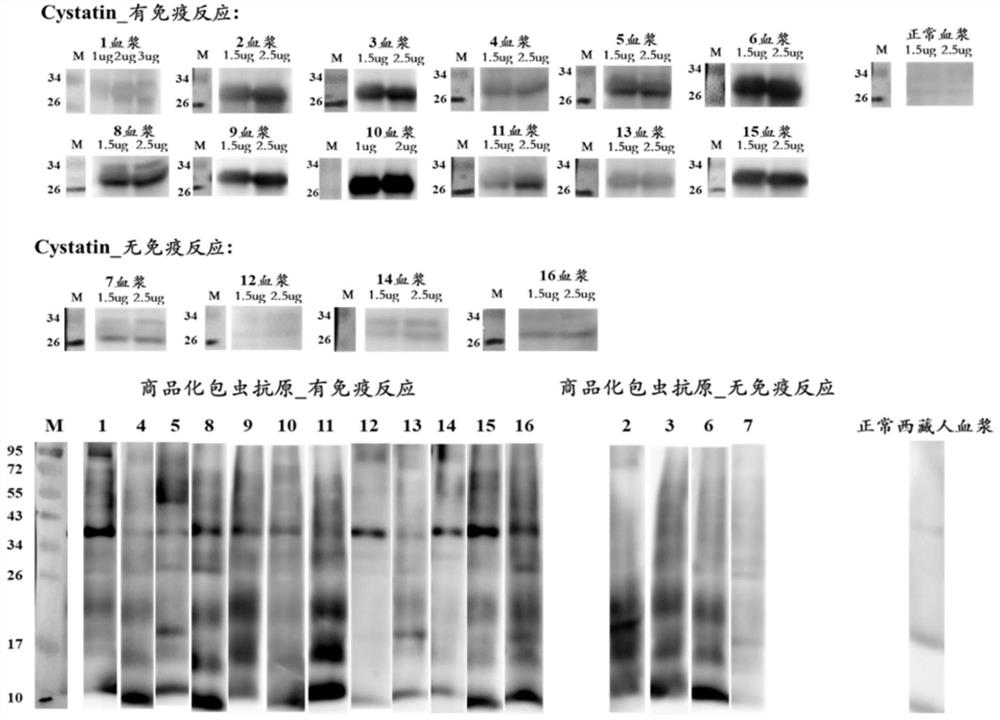
Neoantigen Cystatin protein for echinococcosis
The invention discloses a neoantigen Cystatin protein for echinococcosis and a preparation method of the neoantigen Cystatin protein. The amino acid sequence of the Cystatin protein provided by the invention contains all or part of the following components: the 1st to 25th sites, the 32nd to 56th sites, the 64th to 84th sites, the 89th to 95th sites, the 141st to 147th sites and the 155th to 168th sites of SEQ ID No.1. The neoantigen Cystatin protein is found through a GeLS-MS / MS technology, and the neoantigen Cystatin protein for echinococcosis can be used for manufacturing an ELISA kit and used for clinically detecting human echinococcosis. The Cystatin protein is used for detecting plasma of 14 cases of postoperative echinococcosis people and plasma of 6 cases of normal Tibetan people, the positive detection rate is 93%, and the negative detection rate is 100%. According to the invention, a hydatid antigen library is expanded, and a certain contribution is made to diagnosis of human-derived echinococcosis.
Owner:BGI GENOMICS CO LTD
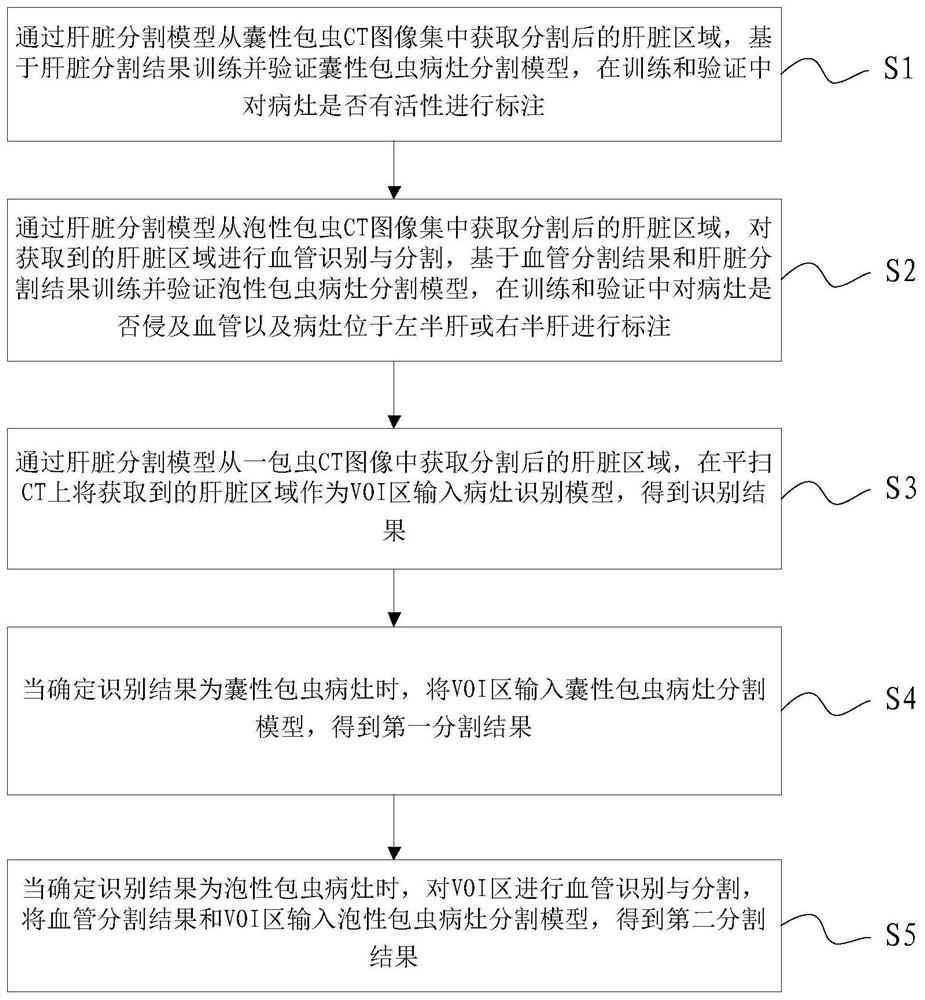
A Neural Network-Based Segmentation Method and System for Hepatic Hydatid Lesions
ActiveCN109685809BImprove diagnostic efficiencyImprove diagnostic accuracyImage enhancementImage analysisHepatic EchinococcosisComputer vision
The invention discloses a neural network-based method and system for segmenting hepatic echinococcosis lesions. The method includes: S1, training and verifying the segmentation model of cystic echinococcosis lesions; S2, training and verifying the segmentation model of alveolar echinococcosis lesions; S3, Obtain the segmented liver area from a hydatid CT image, input the liver area into the lesion recognition model, and obtain the recognition result; S4. When the recognition result is determined to be a cystic hydatid lesion, input the VOI area into the cystic hydatid lesion segmentation model to obtain the first segmentation result; S5. When it is determined that the recognition result is an alveolar echinococcosis lesion, perform blood vessel identification and segmentation on the VOI area, and input the vessel segmentation result and the VOI area into the alveolar echinococcosis lesion segmentation model to obtain the second Split results. The method and system provided by the present invention perform fusion recognition and feature extraction on multi-modal medical images through various models, assist doctors in screening echinococcosis, and improve diagnostic efficiency and accuracy.
Owner:TSINGHUA UNIV
Application of dehydrocamelinarum derivatives as preparation of medicaments for the treatment of cystic hydatid disease
ActiveCN105998014BLow toxicityLower survival rateOrganic active ingredientsOrganic chemistryHarmineEfficacy
The invention relates to the technical field of application of a harmine derivative, and particularly discloses the application of a harmine derivative to preparation of drugs for treating cystic echinococcosis for the first time. In-vitro pharmacodynamic experiment data prove that the harmine derivative can reduce the survival rate of echinococcosis granulosis cyst remarkably; In-vivo pharmacodynamic experiment data prove that the efficacy of the harmine derivative and the efficacy of albendazole are similar in treating cystic echinococcosis; in-vitro-and-vivo safety evaluation experiment data prove that the toxicity of the harmine derivative is lower than that of a harmine monomer with cystic echinococcosis resisting activity. Therefore, a new approach is provided for treating cystic echinococcosis.
Owner:THE FIRST TEACHING HOSPITAL OF XINJIANG MEDICAL UNIVERCITY +1
Harmine gastrointestinal tract adhesive tablet and preparation method thereof
ActiveCN111700867AImprove adhesionConvenient treatmentOrganic active ingredientsPill deliveryLactoseTableting
The invention discloses a harmine gastrointestinal tract adhesive tablet and a preparation method thereof. The adhesive tablet comprises the following components in percentage by mass: 30-35% of harmine, 10-12% of chitosan, 15-20% of lactose, 8-10% of an adhesive, 5-8% of an excipient, 8-10% of a lubricant and 8-10% of a flow aid. The preparation method comprises the following steps of firstly, uniformly mixing the harmine, the chitosan and the lactose, adding the excipient, and performing drying to obtain dry powder; secondly, uniformly mixing the adhesive, the lubricant and the flow aid, anduniformly mixing a mixture with the dry powder to obtain a tabletting mixture; and finally, putting the tabletting mixture into a tabletting device, and pressing the mixture under the pressure of 40-60 N to obtain the harmine gastrointestinal tract adhesive tablet. The obtained adhesive tablet has relatively high release rate and adhesive force, can be stably adhered to the gastrointestinal tracts and stably releases medicinal components after being taken, and has a relatively good effect on treatment of echinococcosis.
Owner:SICHUAN ACADEMY OF MEDICAL SCI SICHUAN PROVINCIAL PEOPLES HOSPITAL
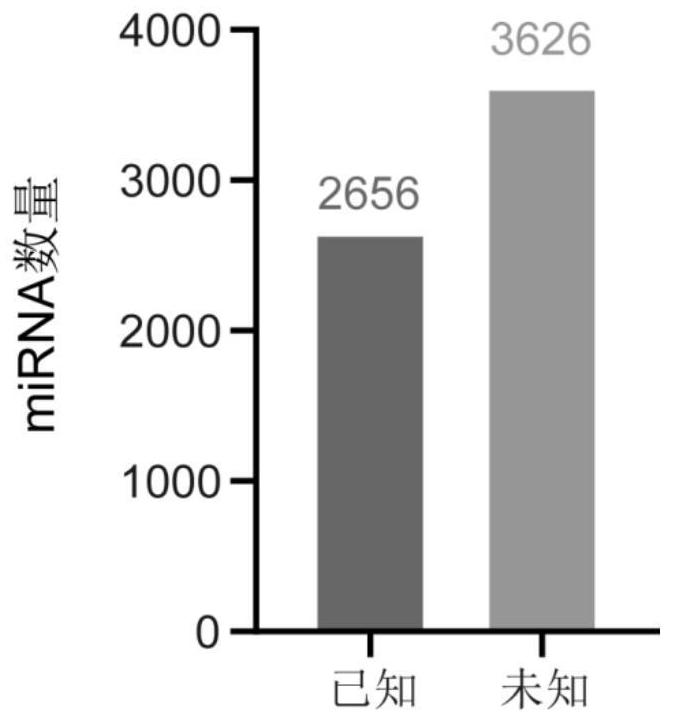
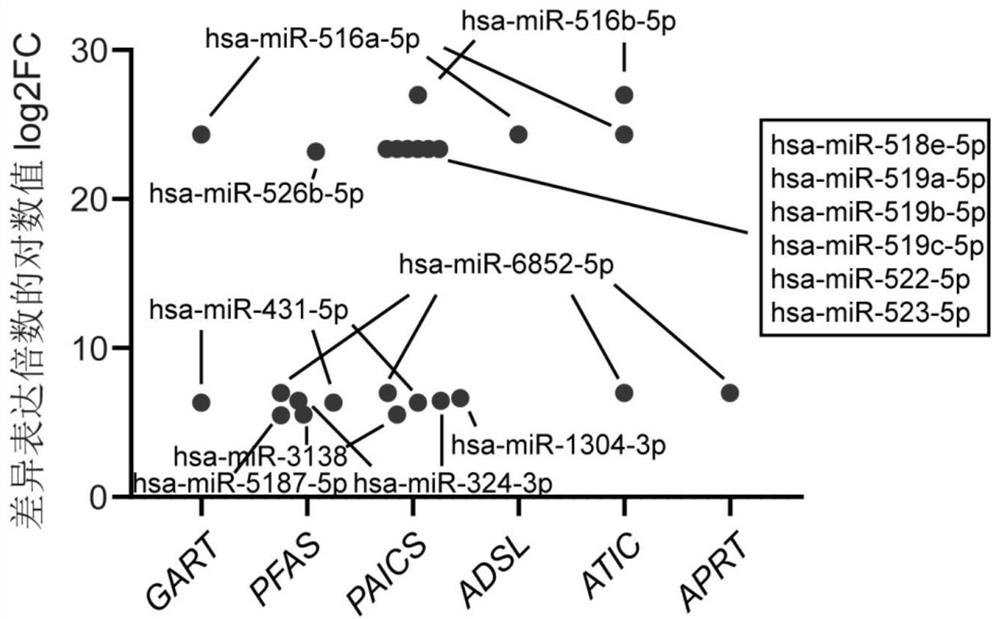
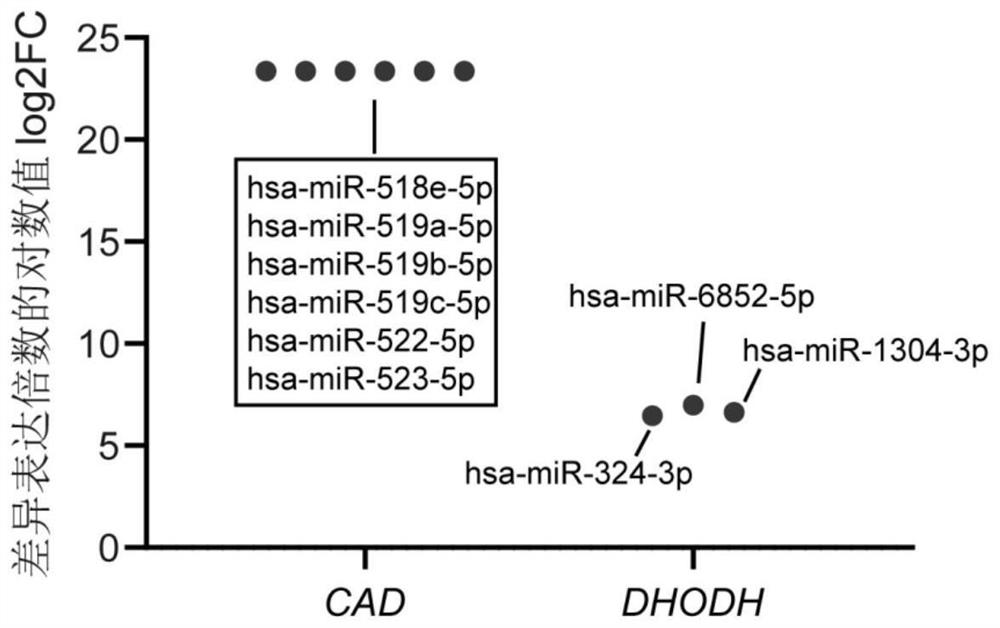
Application of miRNA in preparation of echinococcosis risk assessment or early screening reagent
PendingCN114854848AHigh sensitivityImprove featuresMicrobiological testing/measurementAgainst vector-borne diseasesSerum markersSerum mirna
The invention belongs to the field of medicines, and particularly relates to application of miRNA in preparation of an echinococcosis risk assessment or early screening reagent. The invention relates to a serum miRNA (micro Ribonucleic Acid) marker related to risk assessment or early screening of echinococcosis. The serum miRNA marker is one or more of hsa-miR-516a-5p, hsa-miR-516b-5p, hsa-miR-518e-5p, hsa-miR-519a-5p, hsa-miR-519b-5p, hsa-miR-522-5p, hsa-miR-523-5p, hsa-miR-6852-5p, hsa-miR-1304-3p, hsa-miR-3138, hsa-miR-431-5p, hsa-miR-324-3p, hsa-miR-526b-5p and hsa-miR-5187 As a novel biomarker, the miRNA disclosed by the invention is good in stability and high in sensitivity and specificity.
Owner:NANJING MEDICAL UNIV
Free DNA sequence derived from echinococcus granulosus and application of free DNA sequence
ActiveCN111733254AIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationGeneticsCystic echinococcosis
The invention discloses a free DNA sequence derived from echinococcus granulosus. The length of the free DNA sequence is equal to 107 basic groups and is represented by SEQ ID NO.24. Besides, the invention further discloses a screening method of the free DNA sequence derived from the echinococcus granulosus. The screening method comprises the following steps: (S1) acquiring a large number of freeDNA sequences from a blood sample of a patient with cytic echinococcosis by virtue of a re-sequencing technique; (S2) carrying out comparative analysis by virtue of a bioinformatics method so as to screen out echinococcus granulosus-sourced free DNA sequences; and (S3) carrying out verification and screening in patients with the echinococcosis and healthy people through a fluorescence quantitativePCR method, so as to obtain the free DNA sequence derived from the echinococcus granulosus. Besides, the invention further discloses application of the free DNA sequence derived from the echinococcusgranulosus in preparation of products for diagnosing and detecting the echinococcosis. A detection result shows that the free DNA sequence has relatively high sensitivity and specificity in detectionof the patient with the echinococcosis and has excellent application prospects in the diagnosis and detection fields of the echinococcosis.
Owner:中国疾病预防控制中心寄生虫病预防控制所国家热带病研究中心 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com