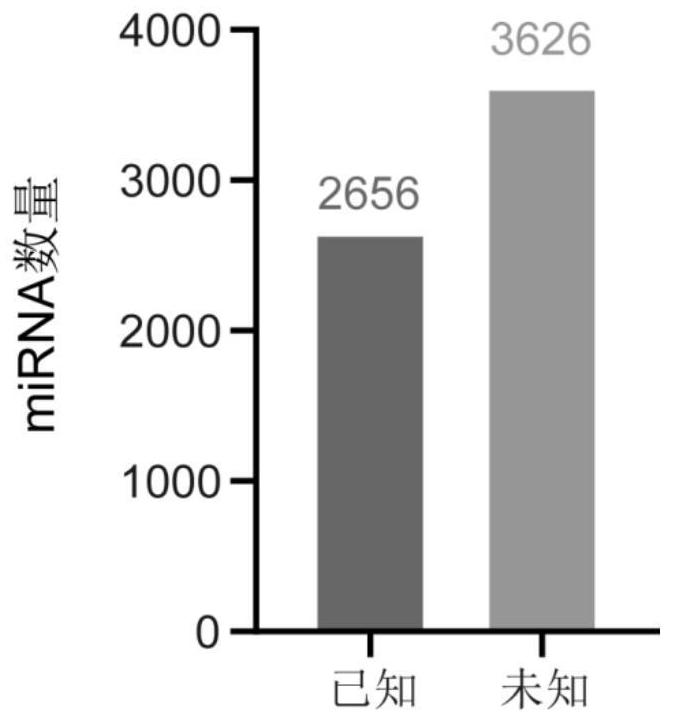
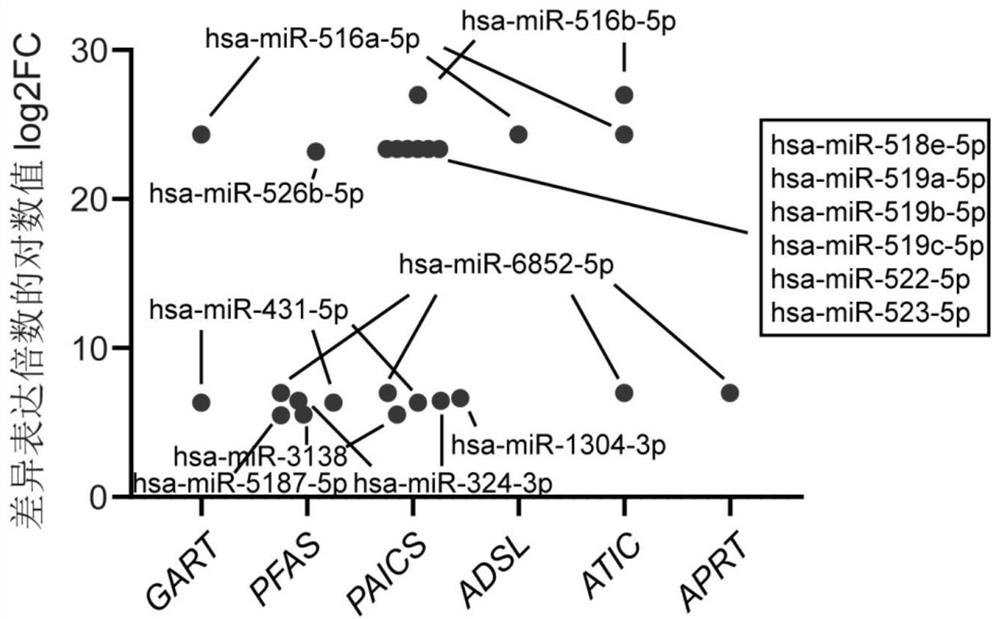
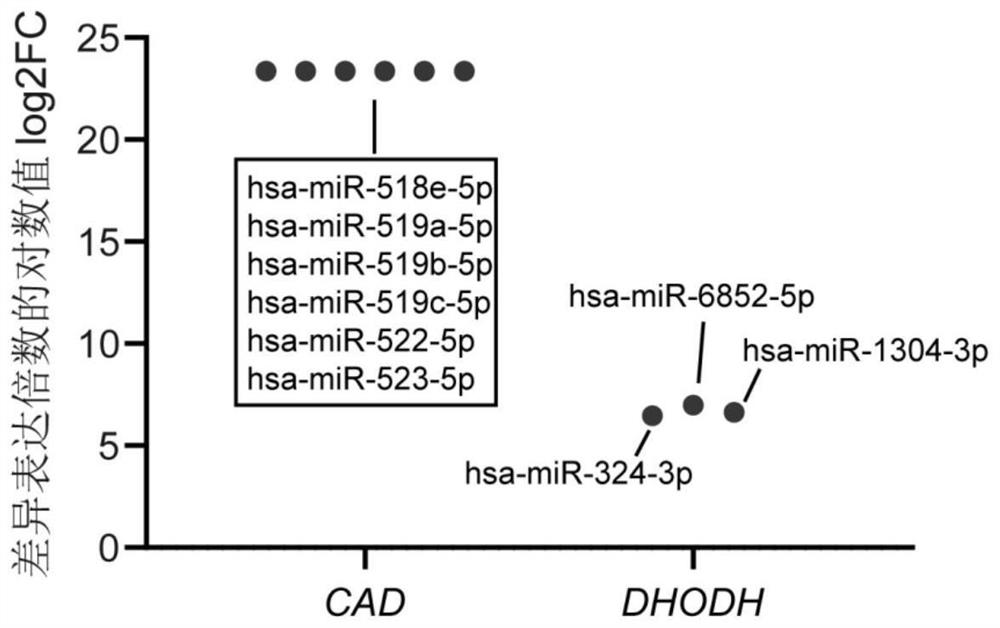
Application of miRNA in preparation of echinococcosis risk assessment or early screening reagent
A technology for risk assessment and echinococcosis, applied in the field of medicine, achieves the effects of good stability, high sensitivity and specificity, and good diagnostic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The present invention will be described in further detail below in conjunction with the embodiments. If the reagents or equipment used are not marked with the manufacturer, they are regarded as conventional products that can be purchased through the market.
[0030] The inventors collected serum from 8 patients with echinococcosis granulosa etiologically confirmed patients (ie, patients with hydatid disease) from the Department of Hepatobiliary Surgery, General Hospital of Ningxia Medical University, and 8 healthy controls provided by the echinococcosis laboratory of Ningxia Medical University. serum.
[0031] 1. Experimental method:
[0032]1. Use miRNeasy Serum / Plasma Kit to extract total RNA from serum of hydatid patients and healthy controls. The specific operation is as follows: after centrifuging the serum at 3000g for 5 minutes, add 1ml TRIzol and mix by pipetting. Let stand for 10 minutes, add 200ul of chloroform, invert vigorously and mix for 30 seconds, let...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com