Sheep echinococcosis infection-resistant gene engineering subunit vaccine as well as preparation method and application thereof
A subunit vaccine and genetic engineering technology, applied in the field of genetically engineered subunit vaccines against sheep echinococcosis infection and its preparation, can solve the problems of high production costs, increased antigen production costs, and restrictions on popularization and application. Good immune protection, good solubility, and the effect of improving gene structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1. Construction of recombinant expression vector of pET28b-2EG95 subunit vaccine against echinococcosis infection
[0037] 1.1 Configuration of solution and medium
[0038] Solution I: 25mmol / L Tris-HCl (pH8.0), 10mmol / L EDTA.
[0039] Solution II: 0.2mol / L NaOH, 1% SDS (prepared and used immediately).
[0040] Solution III: 100mL system, 80mL 5mol / L potassium acetate, 12mL glacial acetic acid, 8mL ddH2O.
[0041] TAE (50×): 242g Tris base, 57.1mL glacial acetic acid, 100mL0.5mol / LEDTA (pH8.0), dilute to 1000mL with sterile water.
[0042] LB medium: tryptone 10g / L, yeast powder 5g / L, sodium chloride 10g / L, adjust the pH value to 7.0 (solid medium contains 1.5% agar).
[0043] Protein loading buffer (2×): 100mmol / L Tris-HCl (pH6.8), 200mmol / L dithiothreitol (DTT), 4% SDS, 0.2% bromophenol blue, 10% glycerol.
[0044] 29 g of 30% acrylamide solution: acrylamide, 1 g of N, N'-methylene acrylamide, dissolved in 100 mL of water, filtered.
[0045] Coomassie bri...
Embodiment 2
[0073] Example 2, pET28b-2EG95 recombinant plasmid induces expression in Escherichia coli BL21 (DE3)
[0074] Transform the pET28b-2EG95 recombinant plasmid into BL21(DE3) Escherichia coli, and spread the LB solid plate (the method is the same as "1.5"). Through monoclonal screening, high-quality and high-yield strains were obtained. Then inoculate the high-yield strain into 500mL LB medium (containing ampicillin and chloramphenicol), and culture it with shaking at 37°C until the OD value is about 1.5-2.0, then inoculate the seed liquid into the fermenter for For fermentation, when the OD value of the bacteria reaches about 25-30, the temperature is lowered to 20°C, and when the temperature is stable, IPTG is added to a final concentration of 0.4mM, and induced for 12-16 hours.
Embodiment 3
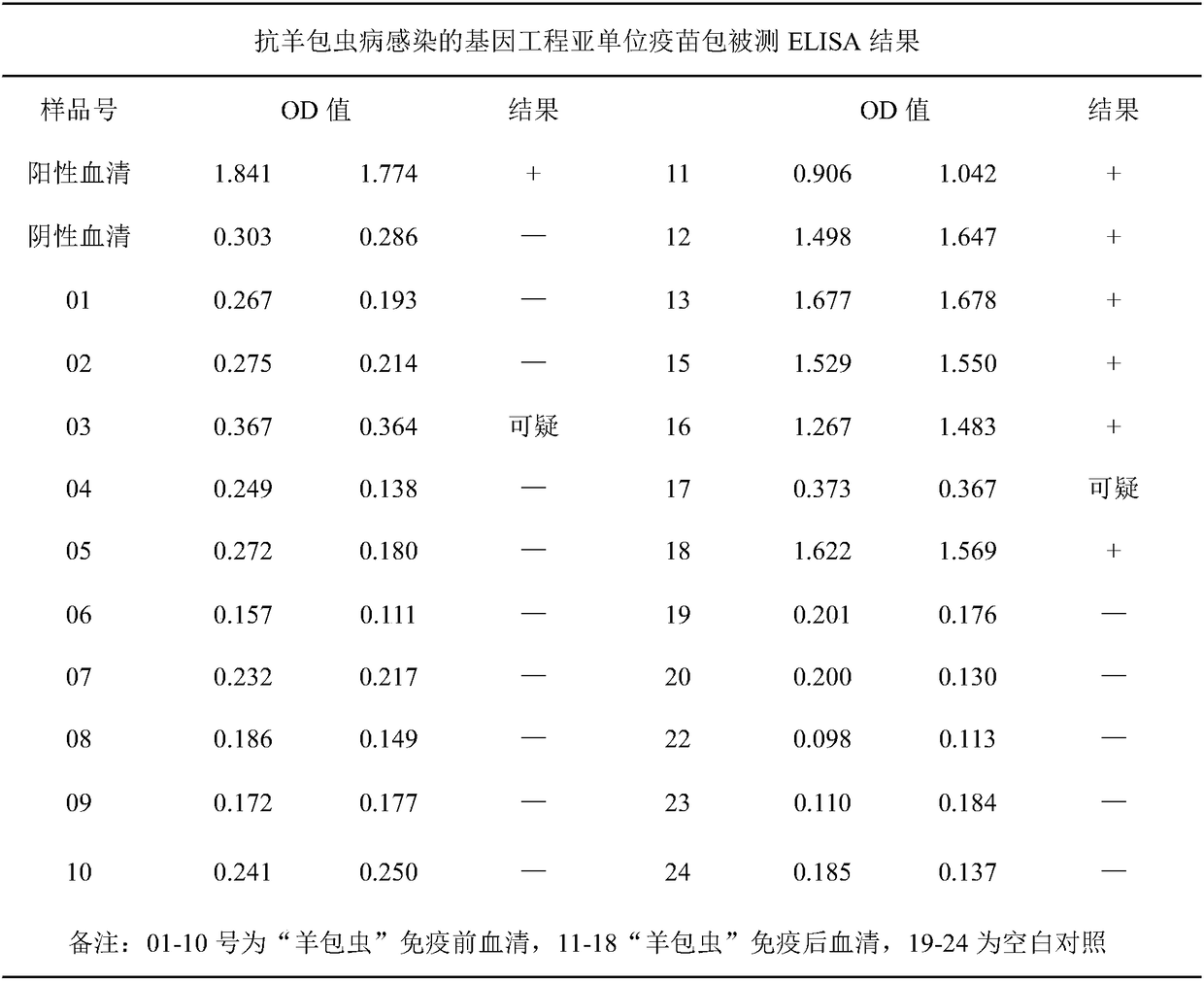
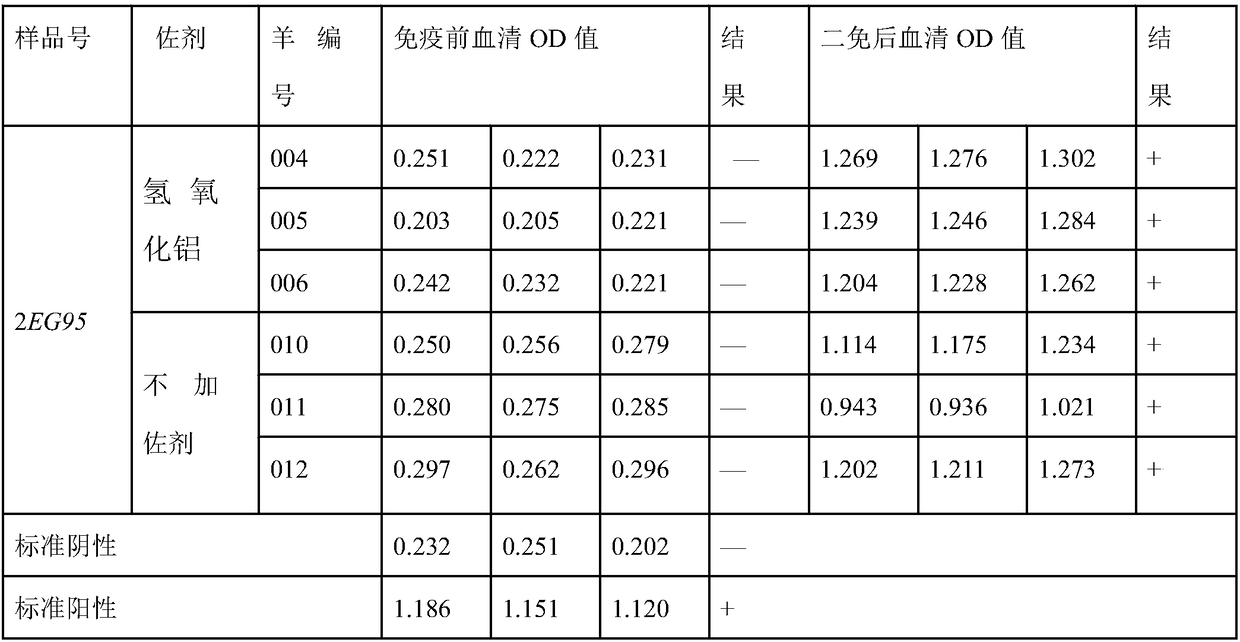
[0075] Embodiment 3, the purification of hydatid hydatid 2EG95 protein
[0076] Buffer Z: 25mM Tris-HCl (pH7.2), 25mM Potassinm Phosphate (pH6.8), 500mM NaCl, 10% Glycerol, 1mM DTT
[0077] Equilibrium buffer: 10mM Tris-HCl (pH7.2), 250mM NaCl, 10% Glycerol
[0078] 1M imidazole, pH 7.0
[0079] 3.1 Fermentation broth centrifugation and whole cell disruption
[0080] After the fermentation, the fermentation broth was centrifuged (10,000 rpm for 8 min), the supernatant was discarded, and the precipitate was resuspended with BufferZ. The resuspended bacteria were crushed with a high-pressure homogenizer (crushing pressure: 1000 bar), centrifuged after crushing (23000 rpm for 1 h), and the supernatant was collected.
[0081] 3.2 Purification of target protein
[0082] Add 3-5% imidazole to the crushed supernatant, first use 100% Equilibrium buffer to equilibrate the Ni-NTA chromatography column, then hang the sample, and directly use 25% imidazole to elute the target protein ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com