Neoantigen Cystatin protein for echinococcosis
A protein and amino acid technology, applied in the direction of resistance to vector-borne diseases, peptide sources, instruments, etc., can solve problems such as difficulty in protein selection, lack of specificity and/or sensitivity, and difficulty in separating echinococcosis proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1, the acquisition of echinococcosis neoantigen Cystatin protein
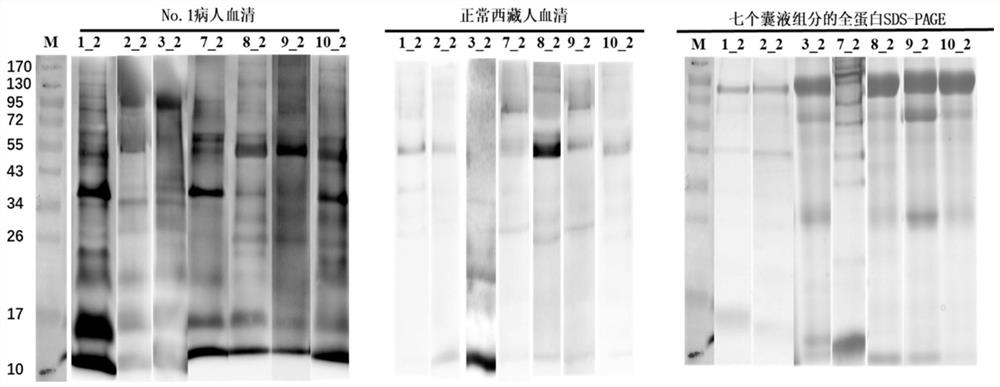
[0059] 1. The process of identifying more hydatid proteins from hydatid cysts isolated from hydatid patients after surgery
[0060] (1) Firstly, the hydatid cysts separated from 6 hydatid patients were divided into four parts according to their tissue structure to extract protein. Whether the cyst fluid is transparent or not, the 6 hydatid cysts were divided into a transparent group and an opaque group.
[0061] (2) The protein was extracted from the four components of each hydatid cyst, and the extracted protein was subjected to liquid enzymatic hydrolysis, and then identified by LC-MS / MS using a QE mass spectrometer.
[0062] (3) The raw data of QE off-machine was carried out using the maxquant protein identification software and the collection of human and hydatid protein databases (Unreviewed (TrEMBL) database downloaded from the Uniprot (https: / / www.uniprot.org / ) website) Hydatid protei...
Embodiment 2
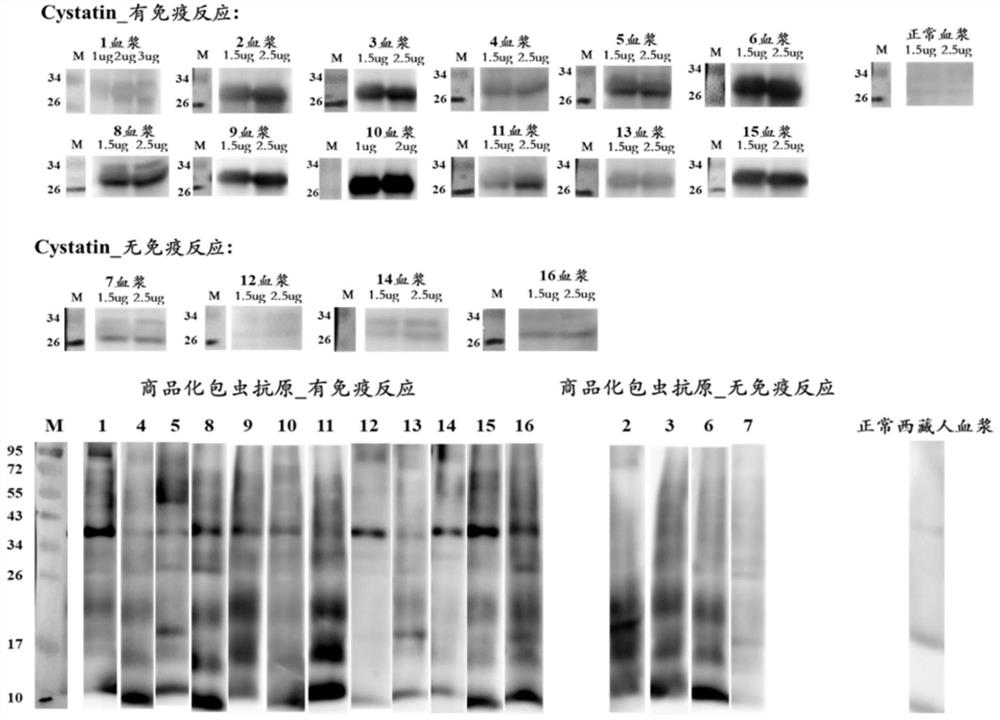
[0106] Example 2, ELISA experimental verification case of echinococcosis recombinant antigen Cystatin recombinant protein
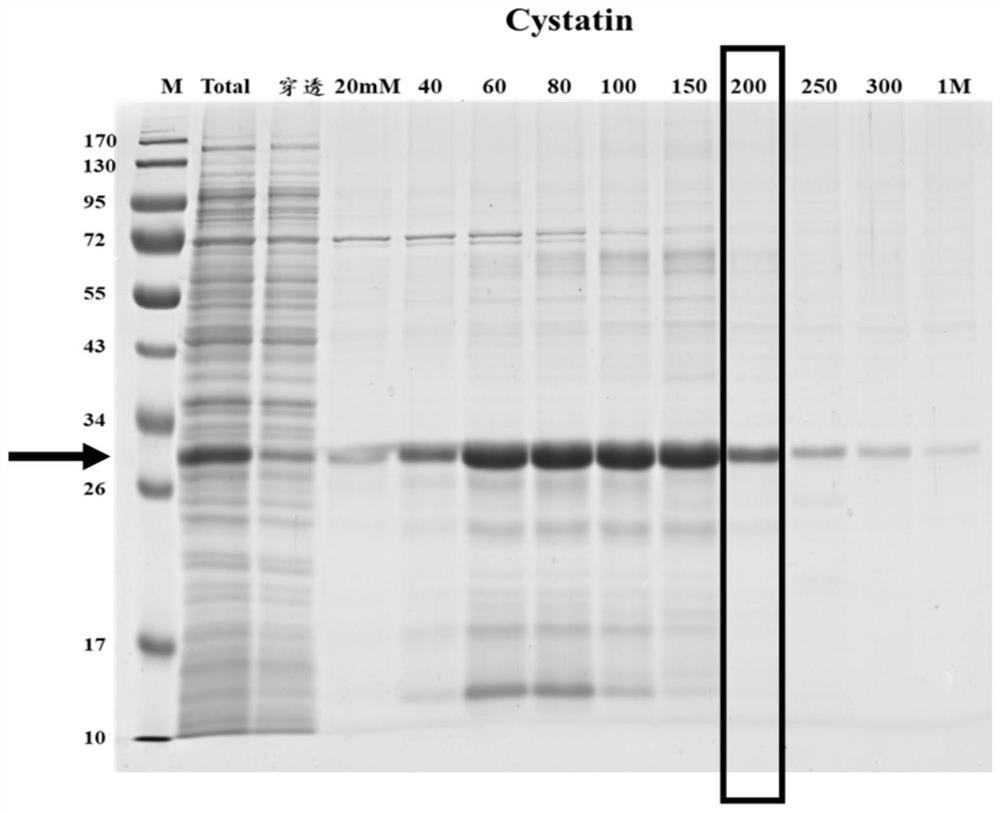
[0107] The detected objects were 14 postoperative echinococcosis patient plasma (clinically confirmed as positive) and 6 cases of normal Tibetan plasma (clinically confirmed as negative), which were carried out according to the standard operating procedures of indirect ELISA, and the results showed that Cystatin recombinant protein (purification gained in Example 1, i.e. figure 2 The target protein eluted when the middle imidazole concentration is 200mM) the positive detection rate is 93%, and the negative detection rate is 100%; Minuo Biotechnology Co., Ltd., product number: YM-VI08) when testing the same plasma, the positive detection rate was 86%, and the negative detection rate was 100%.
[0108] 1. ELISA experiment specific operation steps
[0109] (1) Antigen quantification: Each antigen was blown evenly with a pipette gun before coating, and a m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com