Echinococcus granulosus recombinant protein EgG1Y162-2 (4) and application thereof
A technology of Echinococcus granulosus and recombinant protein, applied in the field of bioengineering, can solve the problems of non-immunogenicity, achieve good immunogenicity and specificity, and promote maturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
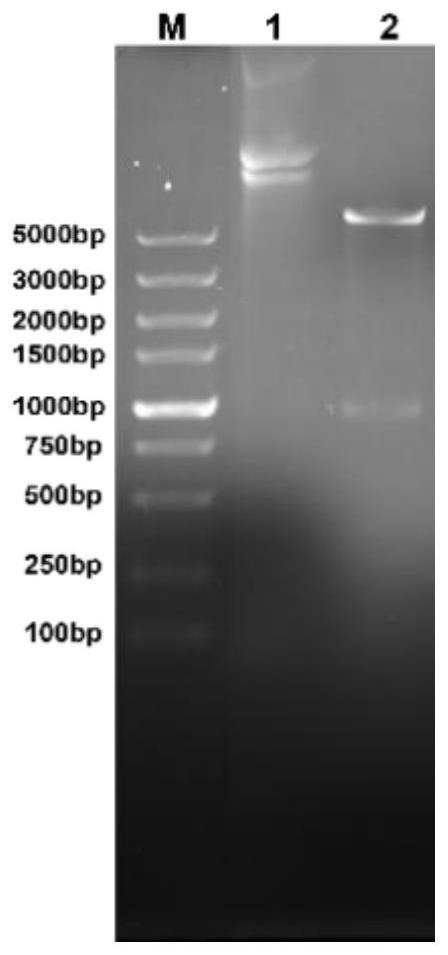
[0032] Example 1 Construction and identification of PET30A-EGG162-2 (4)
[0033] Specific implementation method: Permid enzyme cutting identification for the plasmid PET30A-EGG162-2 (4) synthesized by Shanghai Shengong PET30A-EGG162-2 (4) Use 1 % agar gel electrophoretic and swimming. figure 1 Essence
Embodiment 2
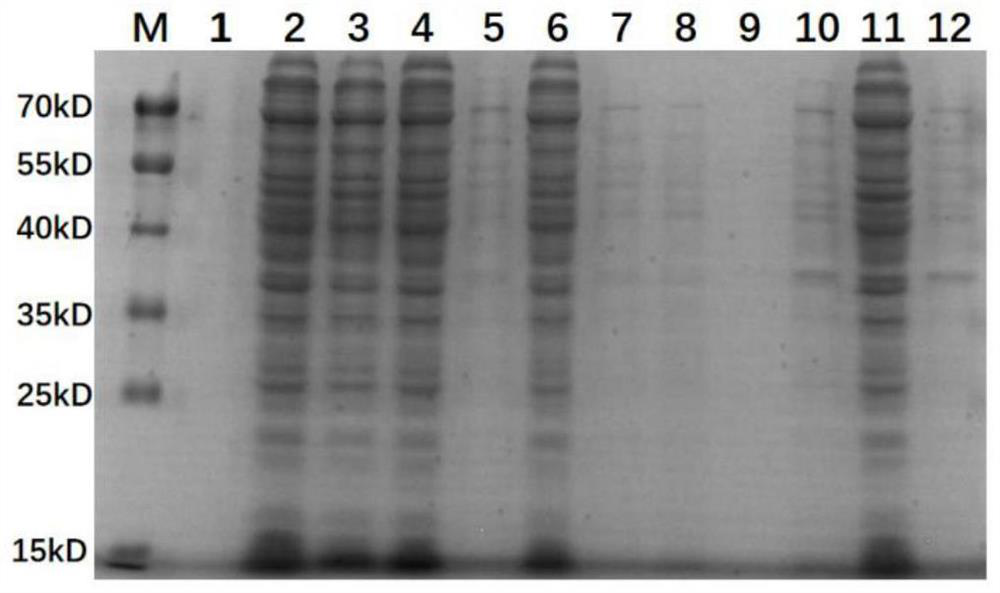
[0034] Example 2 Reorganization protein Egg162-2 (4) induction expression
[0035]Specific implementation method: ribbon expresses the plasmid PET30A-EGG1Y162-2 (4) Converted in ECOLI.BL21 (DE3), picked a single cloning inoculation in the LB liquid medium of 30 μg / ml of 30 μg / ml of Kenamycin. 37 ° C, 220R / min shake the bed oscillation and cultivate overnight. The next day, the medium liquid of this overnight cultivation is inached 1: 50 in the LB liquid medium containing 30 μg / ml of Kenamycin. When the mining oscillating oscillation is cultivated to 0.6-0.8, the bacteria liquid is installed in 12 50ml sterilized centrifugal tubes, and the average bacterial solution in each centrifugal tube is performed. The centrifugal tube equipped with a bacterial solution is numbered to 1 to 12, and then divided into three groups in the order of numbers. The first group 1, 2, 3, and 4 adds an inductive agent IPTG 0.2mmol / L. The second group of 5, 6, 7, and 8 adds the induction IPTG 0.5mmol / L. T...
Embodiment 3
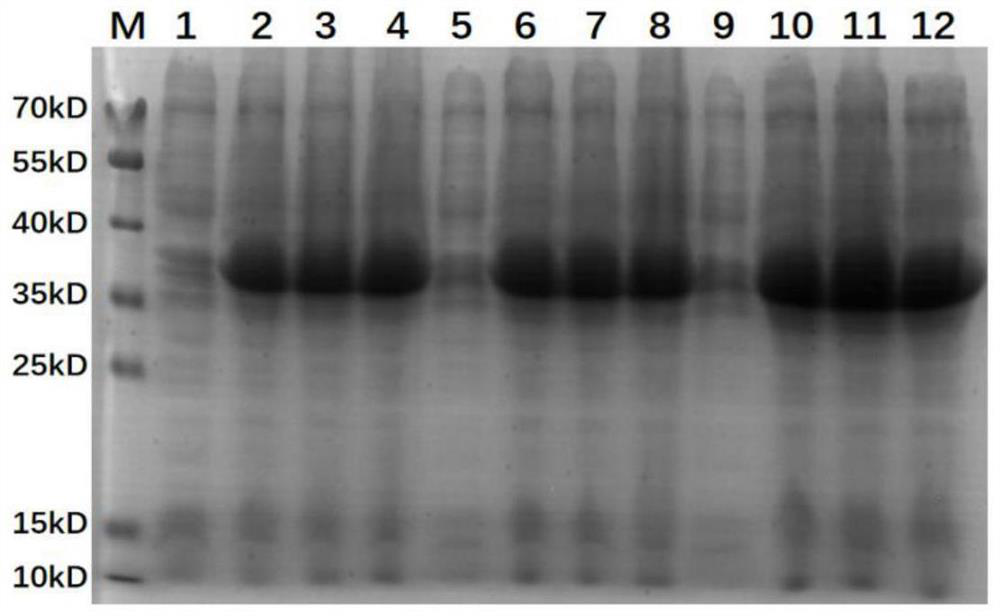
[0036] Example 3 Nickel Pillar Purification Reorganization protein EGG1Y162-2 (4)
[0037] Specific implementation method: According to the exploring reorganized protein EGG1Y162-2 (4) The best condition for inducing, the IPTG concentration is 0.5mmol / L, a large amount of protein is induced under 37 ℃ 4 hours, collects bacteria precipitation, and per gram of bacteria per gram of bacteria Add 7ml of PBS to dilute and mix, add PMSF with a final concentration of 1mm, then perform cell ultrasonic crushing, set up working time 5s, work gap 5s, 3min throughout the process, operate three ultrasound crushing, at 4 ° C, 12000rpm / min centrifuged 10min 10min 10min 10min Get the soluble protein in the Qing Dynasty and purify the protein EGG162-2 (4) purification of the nickel column. First, use a 5x pillar volume to balance the HIS column and set aside to filter the collected protein to remove the excess impurities after removing excess impurities. The liquid over the column again. After the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com