Culture medium and method for inducing human pluripotent stem cells to be differentiated into alveolar cells or alveolar organs
A technology of human pluripotent stem cells and alveolar cells, applied in the field of medium for inducing human pluripotent stem cells to differentiate into alveolar cells or alveolar organoids, can solve the problems of cell separation, immature purification, and difficulty in obtaining aborted fetuses. Gentle, easily accessible effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1 Inducing Human Pluripotent Stem Cells to Differentiate into Definitive Endoderm Cells
[0085] All the cells in the present invention are cultured at 37° C. in a cell incubator containing 5% carbon dioxide.
[0086] The initial cells used in Example 1 were induced human pluripotent stem cells DYR0100 (Cell Bank / Stem Cell Technology Platform, Chinese Academy of Sciences, SCSP-1301).
[0087] 10 mL of mTesR1 (STEMCELL Technologies, 85850) medium was used in a Matrigel (Corning, 356231)-coated 24-well plate to maintain culture of DYR0100. When the confluence of DYR0100 clones reached 90%, differentiation was induced.
[0088] The basal medium used to induce differentiation was LD basal, and the formula of LD basal was: 100 mL DMEM / F12 (Gibco, C1410500BT330500BT) medium was added with 1 mL GlutaMAX (Gibco, 35050061) (100×), 1 mL N2 supplement (Gibco, 17502001) ( 100×), 2 mL of B27 supplement (Gibco, 17504044) (50×), 0.1 mL of ascorbic acid-2-phosphate (Yuan Ye, ...
Embodiment 2
[0091] Example 2 Induction of defined endoderm cells to differentiate into lung precursor cells
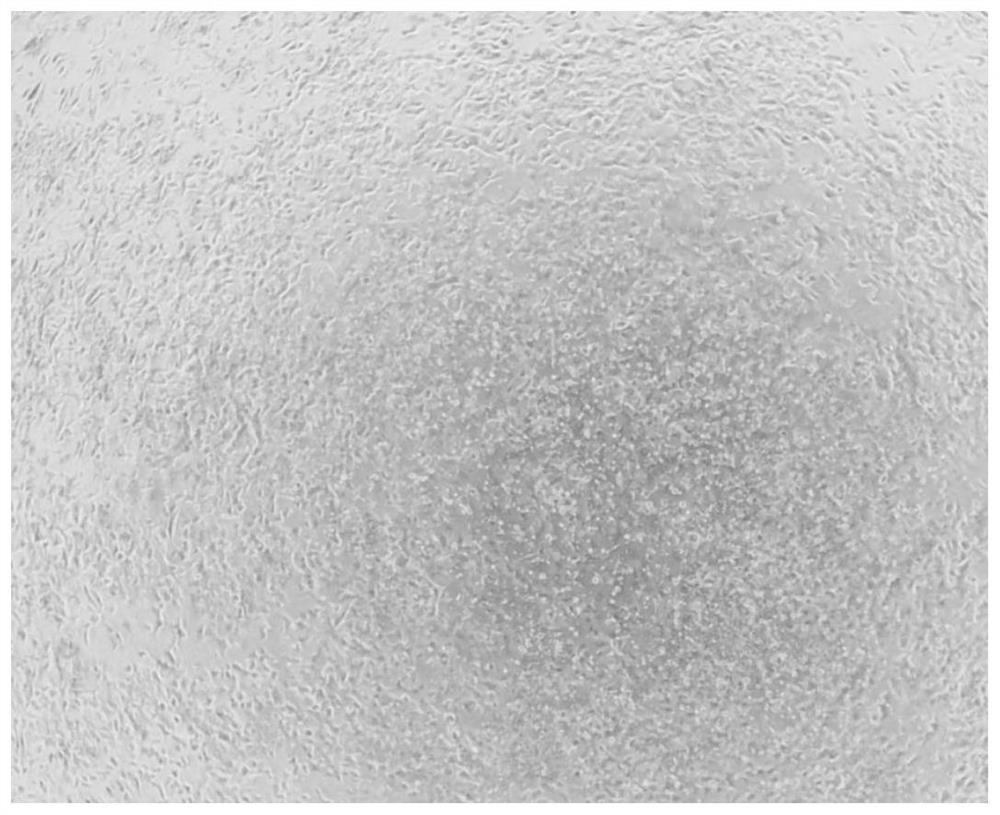
[0092] For the defined endoderm cells finally obtained in Example 1, LDM-3 was used in the first stage to induce differentiation of the defined endoderm cells obtained in Example 1 for 3 days to become front foregut cells. The formula of LDM-3 is: SB431542 (Selleck, S2924) (10 μM) and dorsomorphin (Selleck, S7306) (2 μM) are added to LD basal medium.
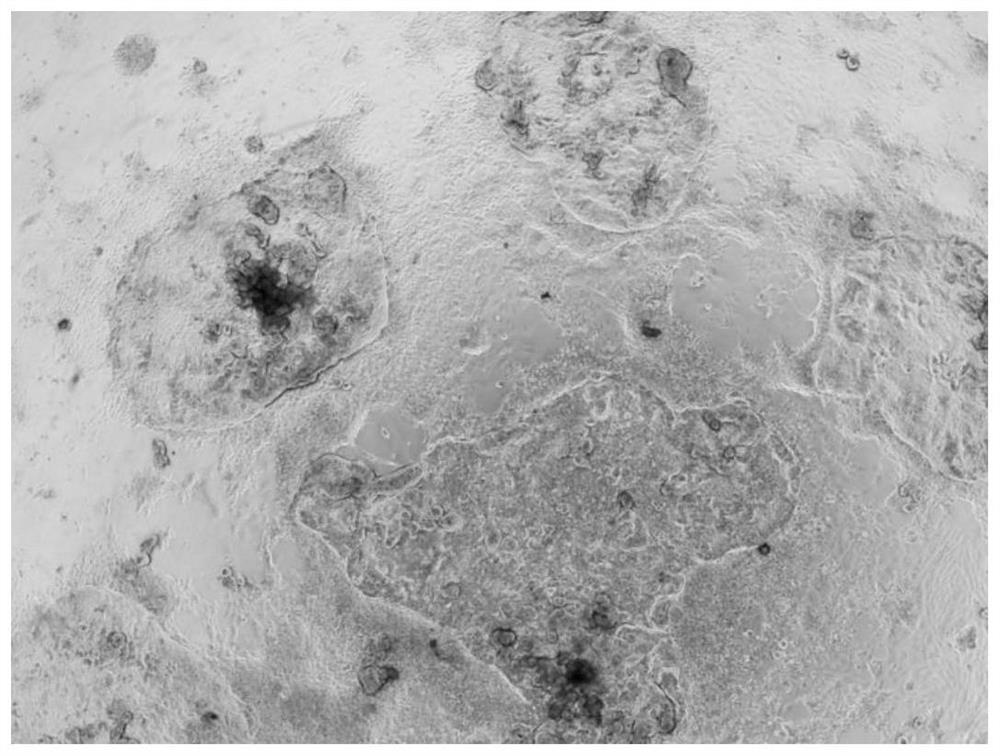
[0093] In the second stage, LDM-4 was used to induce the differentiation of the frontal foregut cells obtained above for 9 days to become lung precursor cells ( figure 2 ). The formula of LDM-4 is: add CHIR99021 (3μM), retinoic acid (Sigma, R2625) (50nM), BMP4 (Gibco, PHC9531) (10ng / mL), FGF7 (R&D, 251-KG- 050) (10 ng / mL), FGF10 (R&D, 345-FG-025) (10 ng / mL).
Embodiment 3
[0094] Example 3 Induces differentiation of lung precursor cells into monolayer alveolar cells
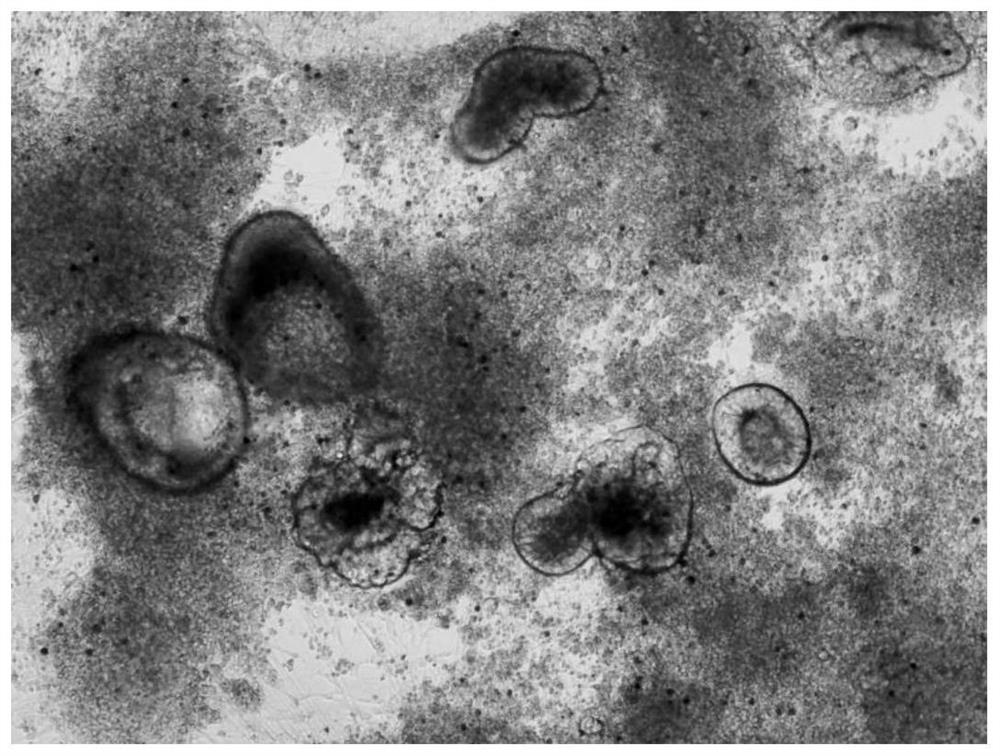
[0095] For the lung precursor cells finally obtained in Example 2, use LDM-5 to induce the differentiation of lung precursor cells for about 16 days, and finally become alveolar cells ( image 3 ). The formula of LDM-5 is: add CHIR99021 (3μM), FGF7 (10ng / mL), dexamethasone (Sigma-Aldrich, D4092) (50nM), cAMP (Sigma-Aldrich, B5386) (0.1mM) to LD basal medium ), 3-isobutyl-1-methylxanthine (Sigma-Aldrich, I5879-250MG) (0.1 mM).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com