Application of polypeptide in preparation of medicine for treating endometriosis
A technology for endometriosis and endometriosis, applied in the field of biomedicine, can solve problems such as large side effects, achieve the effect of relieving abdominal pain and improving endometriosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Establishment of an animal model of endometriosis
[0022] In the study of endometriosis, the acquisition of endometriosis tissue from clinical patients is limited, so it is necessary to establish an animal model of endometriosis. The inventors selected 45 female mature SPF SD rats to establish the endometriosis model. It was identified that 30 SD rats were successfully modeled, with a success rate of 67.7%. The specific animal experiment steps (refer to CN102988401A) are as follows:
[0023] (1) 45 sexually mature SPF healthy unmated female SD rats were obtained from the Animal Center of the First Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine;
[0024] (2) Anesthesia by intraperitoneal injection of 1% sodium pentobarbital (40mg / kg);
[0025] (3) After routine disinfection, a longitudinal incision of about 3 cm was made in the middle of the lower abdomen and 1 cm above the pubic symphysis;
[0026] (4) Find the uterus on the back of t...
Embodiment 2
[0040] Test the therapeutic effect of polypeptide on endometriosis
[0041] Medications used:
[0042] Gestrienone: Gestrienone capsules produced by Beijing Zizhu Pharmaceutical Company, dissolved in normal saline. The polypeptide with the amino acid sequence of SEQ ID NO. 1: synthesized and sequenced by Shanghai Shenggong, and the polypeptide was diluted to 10 μg / kg with normal saline.
[0043] Experimental steps:
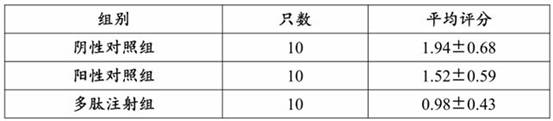
[0044] The 30 SD rats with successful modeling were randomly divided into 3 groups with 10 rats in each group, namely the negative control group (normal saline gavage group), 10 rats, and the positive control group (gestrinone gavage group, 0.5 per day). mg / kg) 10, and the polypeptide injection group (10ml / kg daily) 10. The dosage of normal saline in the control group was the same as that of the drug in the polypeptide injection group for 4 consecutive weeks.
[0045] After 4 weeks, the laparotomy was performed again, and the ectopic endometrial tissue of the co...
Embodiment 3
[0055] Example 3 Clinical trial
[0056] 1 Information
[0057] 1.1 General information
[0058] Selected 40 patients from the First Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine from October 2019 to December 2019, and divided them into two groups by random number table method, namely the treatment group and the control group, with 20 patients in each group. There were no significant differences in gender, age, and course of disease between the groups before treatment, and the groups were well balanced and comparable.
[0059] 1.2 Diagnostic criteria
[0060] According to the standards stipulated by the third academic meeting of the Obstetrics and Gynecology Professional Committee of the Chinese Association of Integrative Medicine and the "Guidelines for Clinical Research of New Chinese Medicines for the Treatment of EM", the following diagnostic criteria are formulated:
[0061] ① progressive dysmenorrhea;
[0062] ②Short abdomen and lumbo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com