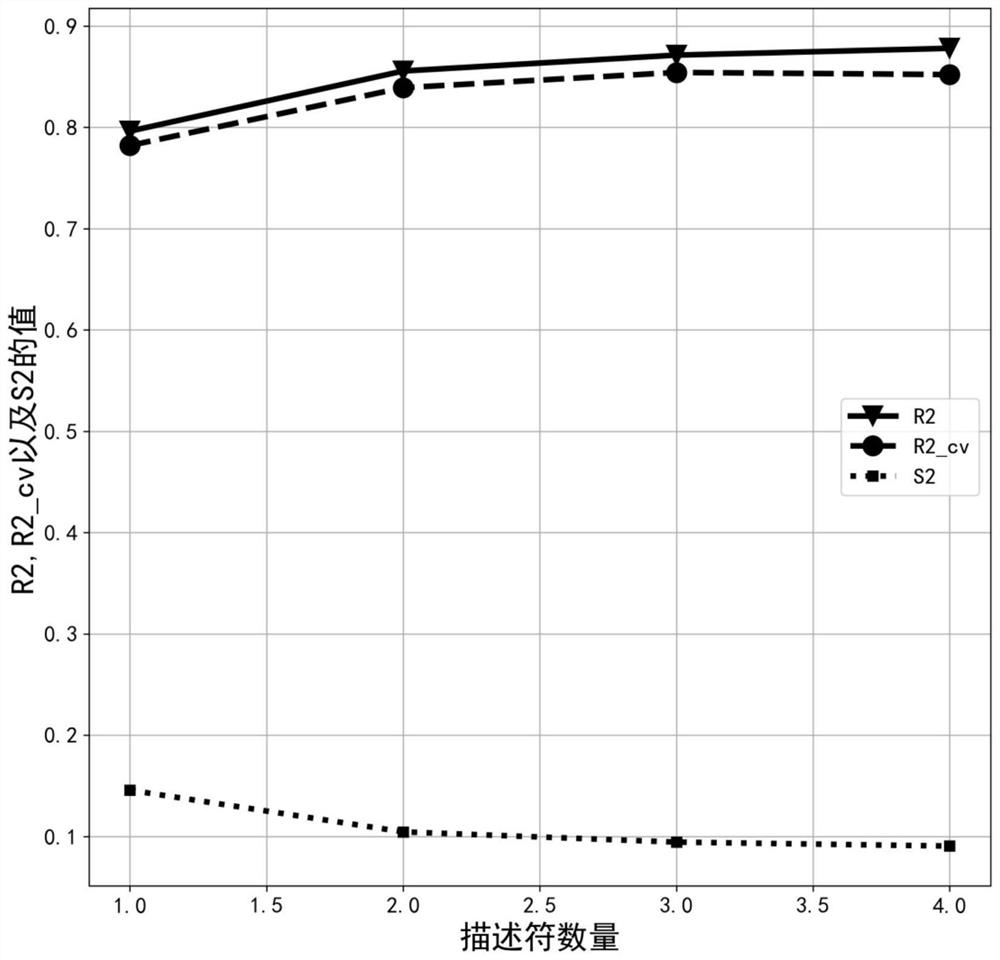
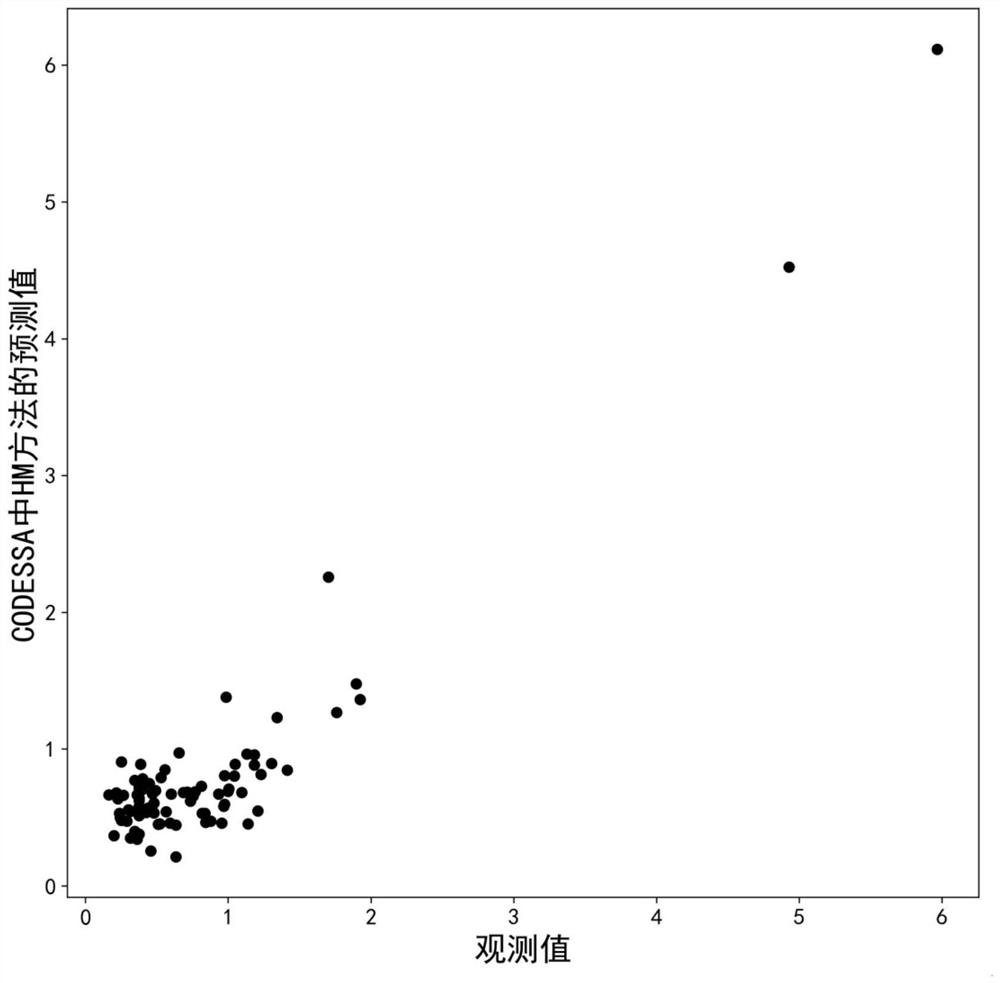
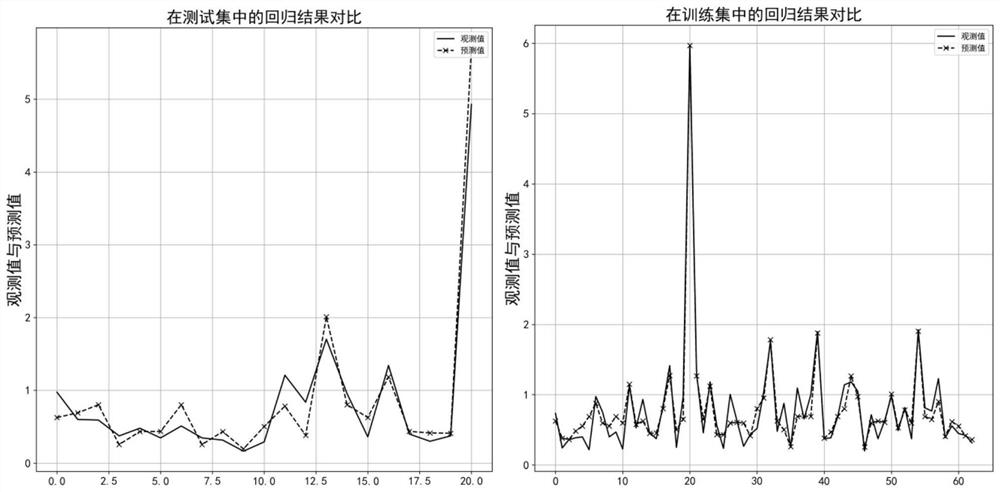
Method for predicting inhibitory activity of fructose-1, 6-diphosphatase inhibitor based on quantitative structure-activity relationship model
A quantitative structure-activity relationship and inhibitory activity technology, applied in computational models, character and pattern recognition, instruments, etc., to achieve the effects of reducing research and development costs, saving research funds and time costs, and reducing risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This example relates to a method for predicting the inhibitory activity of fructose-1,6-bisphosphatase inhibitors based on a quantitative structure-activity relationship model. The specific steps are as follows:
[0034] S1. Collection of sample sets:
[0035]The structures and corresponding inhibitory activities of FBPase enzyme inhibitor molecules were collected; the FBPase enzyme inhibitors were N-arylsulfonyl-indole-2-carboxamide derivatives; specifically: a total of 84 N - Molecular structure of arylsulfonyl-indole-2-carboxamide derivatives and corresponding inhibitory activity, the inhibitory activity is measured by IC50; the standard for collecting inhibitors is: exclude compounds that do not have a specific IC50 value but can only be given a range ;
[0036] S2. Processing and optimization of the sample set:
[0037] To optimize the structure of each inhibitor molecule in the sample set, first use ChemDraw Ultra 8.0 software to draw the 2D structure of each in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com