Monomethyl auristatin E prodrug and preparation method and application thereof
An auristatin and monomethyl technology, applied in the field of monomethyl auristatin E prodrug and its preparation, can solve the problems of large drug side effects, narrow drug treatment window, high chemical toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
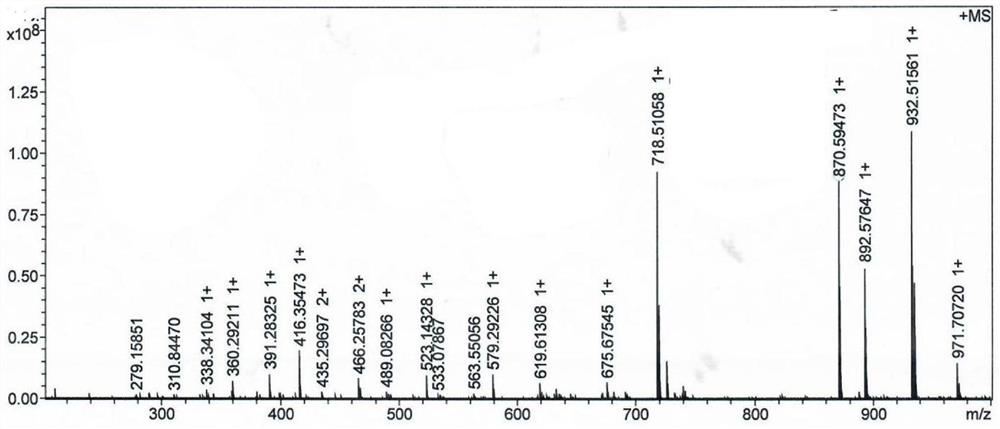
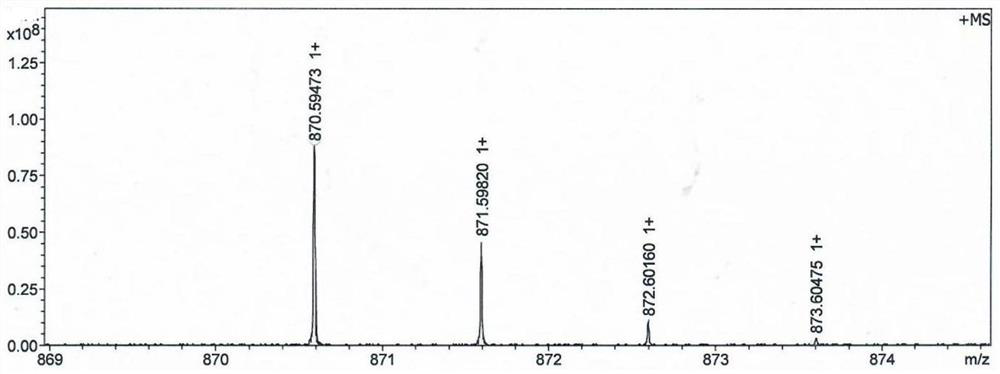
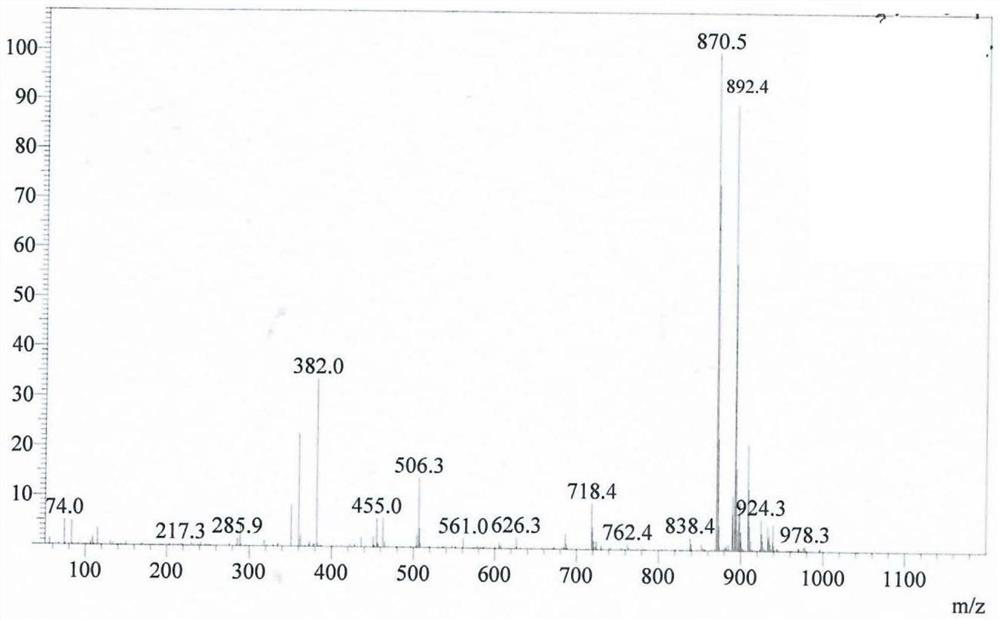
[0055] This example provides a monomethyl Astatin E prodrug-shear type TCO axial isomer -mmae (TCO dax -Mmae, its structure is as follows:
[0056]
[0057] The preparation process is as follows:
[0058] (1) The shear type TCO axial isomer of 1.2: 1 is dissolved in tetrahydrofuran in tetrahydrofuran, and triethylamine is added to tetrahydrofuran, and triethylamine and 4-nitrophenol chlorol. The molar ratio of the methyl ester is 1: 1, 25 ° C for 20 h to obtain a shear type TCO axial isomer to nitrophenyl carbonate;
[0059] (2) The shear type TCO axial isomer of 0.5: 1 of the molar ratio is in the presence of nitrophenyl carbonate and Mmae in dimethylformamide, 1-hydroxybenzene triazole (HOBT), wherein The molar ratio of Hobt and shear TCO axial isomers on nitrophenyl carbonate was 0.5: 1, 35 ° C. Anti-lighting reaction 24h to obtain Mmae prodrug;
[0060] (3) Purification of the MMAE prodrug of the MMAE to obtain a refined product and the yield is 85.5%.
[0061] The resulting...
Embodiment 2
[0063] This example provides a monomethyl Astin E prodrug-shear type TCO plane isomer-MMAE (TCO deq -Mmae, its structure is as follows:
[0064]
[0065] The preparation process is as follows:
[0066] (1) The shear type TCO plane isomer of 1.4: 1 is dissolved in tetrahydrofuran in tetrahydrofuran, and pyridine is added to tetrahydrofuran, and the molar ratio of pyridine and 4-nitrophenol chloroform is added. The reaction was obtained from 1: 1, 25 ° C for 48 hours to obtain a shear type TCO plane isomer to nitrobenzene carbonate;
[0067] (2) The shear type TCO plane isomer of 0.6: 1 in the molar ratio is in the presence of nitrophenyl carbonate and Mmae in dimethylformamide, 1-hydroxybenzene triazole (hobt), where Hobt The molar ratio of the shear type Tco plane isomer to nitrobenzene carbonate is 0.6: 1, 30 ° C. Anti-lighting reaction 24h to obtain Mmae prodrug;
[0068] (3) Purification of the MMAE prodrug of the produced MMAE was purified and the yield was 86.1%.
Embodiment 3
[0070] This example provides a monomethyl Astatin E prodrug-coupling TCO axial isomer-Mmae (TCO lax -Mmae, its structure is as follows:
[0071]
[0072] The preparation process is as follows:
[0073] (1) The coupling TCO axial isomer with a molar ratio of 1.6: 1 is dissolved in tetrahydrofuran in tetrahydrofuran to 4-nitrophenol chlorometry, and the pyridine is added to the molar of pyridine and 4-nitrophenol chloroform. The ratio was 1: 1, 25 ° C for 12h to obtain a coupling TCO axial isomer to nitrobenzyl carbonate;
[0074] (2) A conjugated TCO axial isomer of 0.7: 1 of 0.7: 1 is in the presence of nitrophenyl carbonate and Mmae in dimethylformamide, 1-hydroxybenzene triazole (HOBT), wherein The molar ratio of Hobt and coupling TCO axial isomers on nitrophenyl carbonate is 0.7: 1, 30 ° C. Anti-lighting reaction 36h to obtain Mmae prodrug;
[0075] (3) Conducting the MMAE prodrug of MMAE to obtain a refined product with a yield of 89.8%.
[0076] The resulting MMAE prodrug e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com