Transferrin receptor targeting peptides
A transferrin and receptor technology, applied in the direction of polypeptides, peptides/protein components, peptides containing localization/targeting motifs, etc., can solve the problems of poor response, difficult to use conventional drugs for diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0616]The following embodiments describe non-limiting permutations of combinations of features disclosed herein. Other permutations of combinations of features are also contemplated. In particular, each of these numbered embodiments shall be considered dependent on or related to each preceding or subsequent numbered embodiment, regardless of their order as listed. CLAIMS 1. A composition comprising an engineered transferrin receptor (TfR) binding peptide or variant, homologue, fragment or analog thereof. 2. A composition comprising a peptide construct, wherein the peptide construct comprises: a) a transferrin receptor (TfR) binding peptide or a variant, homologue, fragment or analog thereof; and b) an active agent, wherein said peptide is conjugated to, linked to or fused to said active agent. 3. The composition of any one of embodiments 1-2, wherein the TfR is human TfR, or murine TfR, or both human TfR and murine TfR. 4. A composition comprising an engineered peptide with...
Embodiment 1
[0620] Peptide Manufacturing
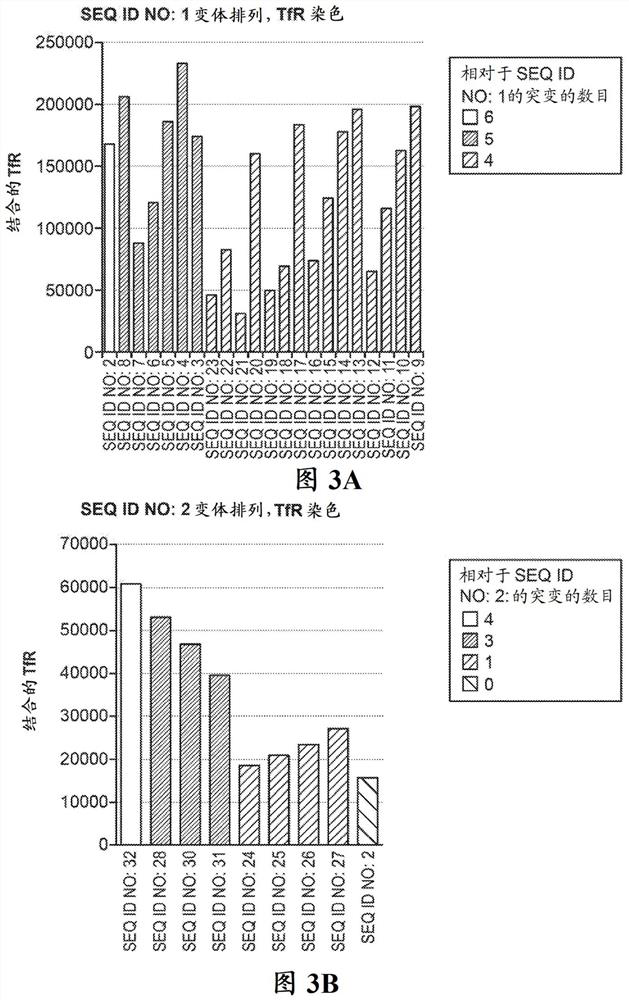
[0621] This example describes the peptides and peptide constructs described herein (e.g., SEQ ID NO: 1 - SEQ ID NO: 70, SEQ ID NO: 136 - SEQ ID NO: 205, SEQ ID NO: 225 - SEQ ID NO: 332, or any of SEQ ID NO:356-SEQ ID NO:361). Published methods were used to produce protein-derived peptides in mammalian cell culture. (A.D.Bandaranayke, C.Correnti, B.Y.Ryu, M.Brault, R.K.Strong, D.Rawlings. 2011. Daedalus: a robust, turnkey platform for rapid production of decigram quantities of active recombinant proteins in human cell lines using novel lentiviral vectors. Nucleic Aci Research. (39) 21, e143).
[0622] The peptide sequence was reverse translated into DNA, synthesized, and cloned in-frame with siderophilin using standard molecular biology techniques (M.R. Green, Joseph Sambrook. Molecular Cloning. 2012 Cold Spring Harbor Press). The resulting construct was packaged into lentivirus, transduced into HEK-293 cells, amplified, isolated by immobilized...
Embodiment 2
[0624] Peptide Expression Using Mammalian Expression Systems
[0625] This example describes the use of mammalian expression systems to express peptides and peptide constructs. Peptides were expressed according to the method described in Bandaranayake et al., Nucleic Acids Res. 2011 Nov;39(21):e143. Tobacco etch virus protease is used to cleave peptides from siderophage (e.g., SEQ ID NO: 1 - SEQ ID NO: 70, SEQ ID NO: 136 - SEQ ID NO: 205, SEQ ID NO: 225 - SEQ ID NO: 332, or Any one of SEQ ID NO:356-SEQ ID NO:361), and by carrying out reverse-phase HPLC (RP-HPLC) with the gradient of acetonitrile and 0.1% TFA aqueous solution to purify, then aliquot and lyophilize for slightly used later. Molecular weights were verified by mass spectrometry.
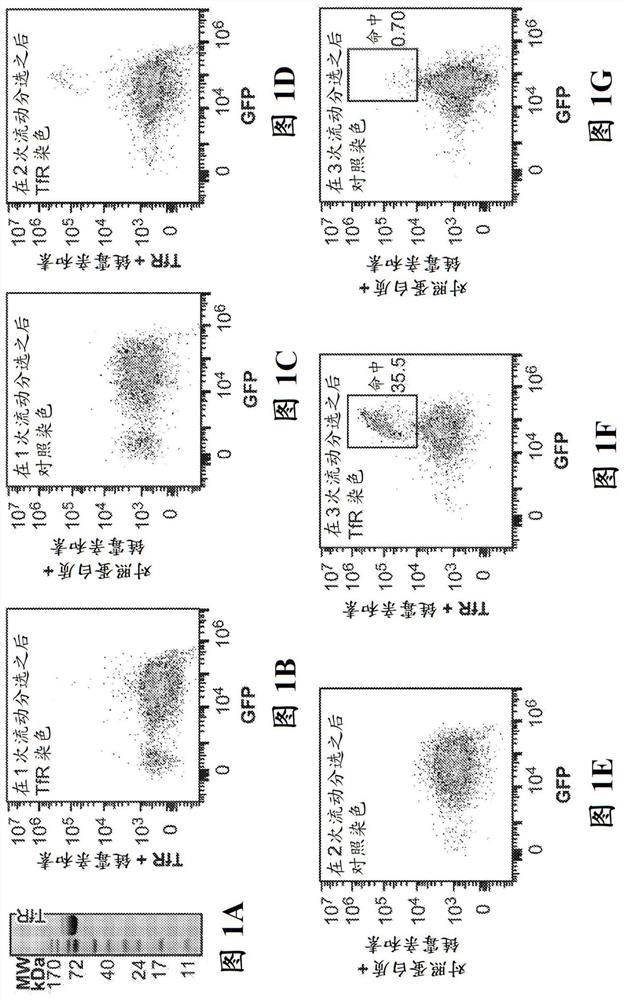
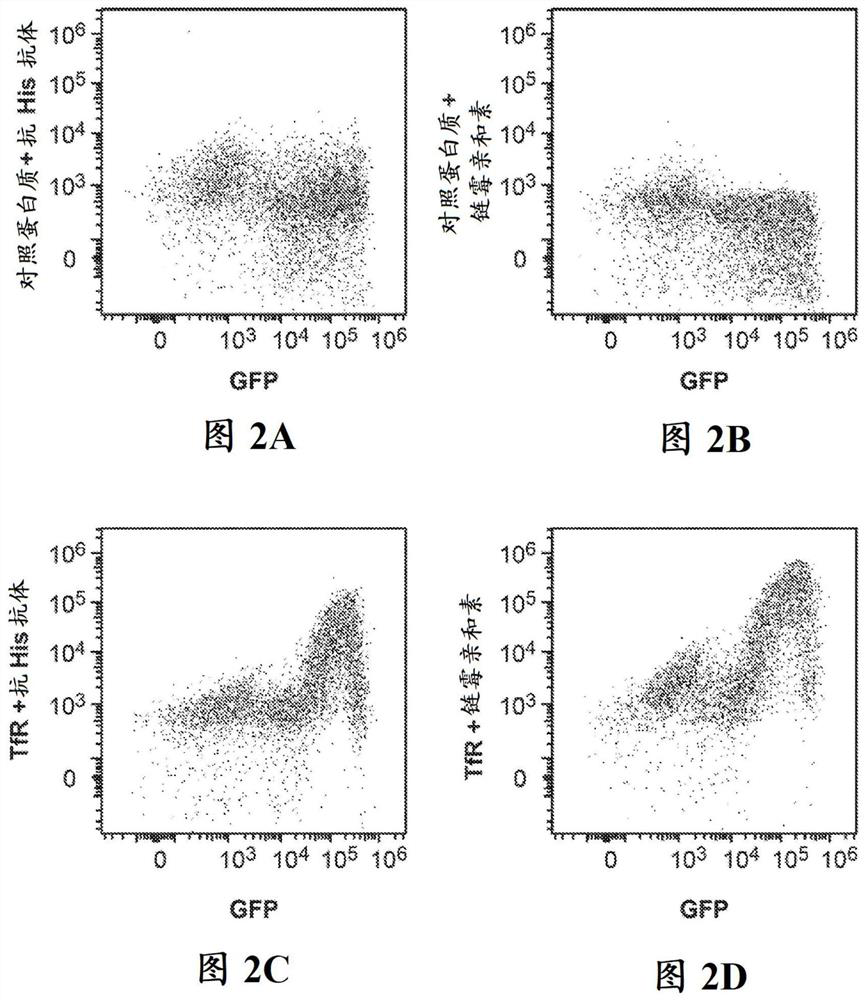
[0626] To optimize and validate the screening method and to identify TfR-binding peptides, the transferrin receptor (TfR) ectodomain ("soluble TfR", SEQ ID NO:353) was cloned into the Daedalus soluble protein producing lentiviral vecto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com