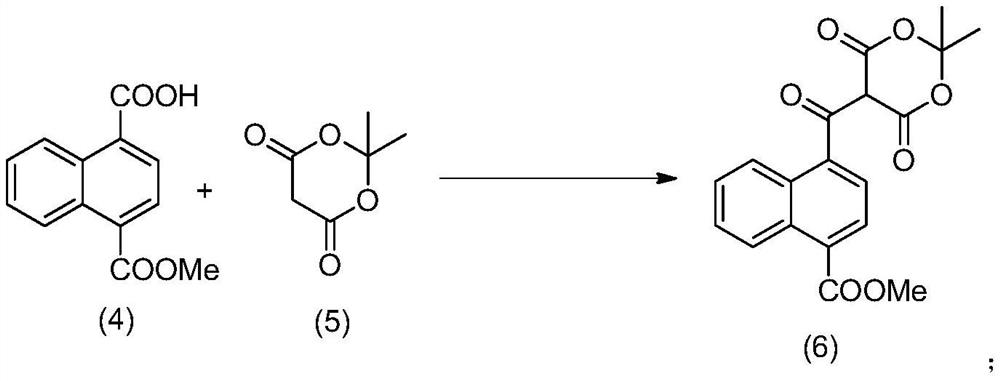
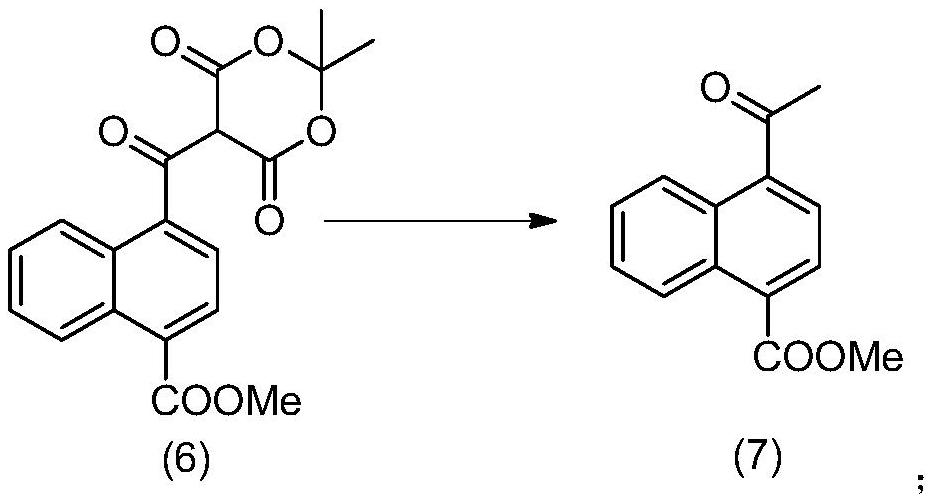
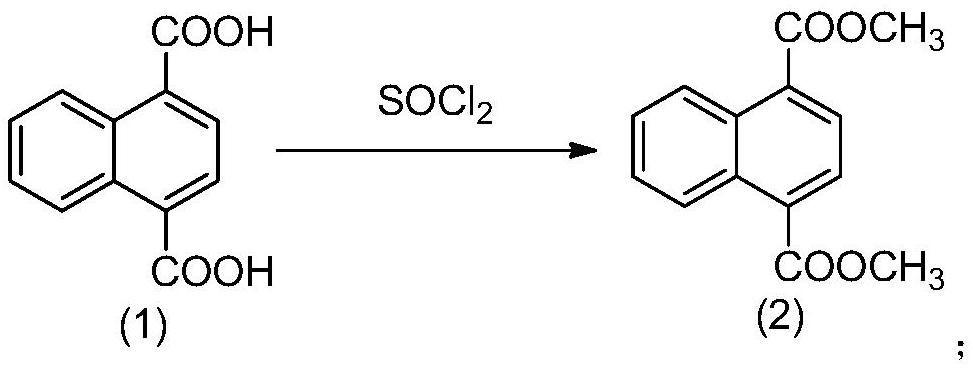
Method for preparing 4-acetyl-1-naphthoic acid
A technology of naphthoic acid and acetyl group, applied in the field of preparing 4-acetyl-1-naphthoic acid, can solve the problems of high cost, low yield, low product yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Preparation of Example 1 Compound (2)
[0081]
[0082] Compound (1), namely 1,4 naphthalene dicarboxylic acid (80 g, 370 mmol) and methanol (560 mL) were added to a 1000 mL round-bottomed flask, the temperature was lowered to below 0 °C, and SOCl was slowly added dropwise with stirring. 2 (92.4 g, 777 mmol), after the dropwise addition was completed, the temperature was raised to 75° C., and the reaction was refluxed for 12 h. After the reaction was completed, it was lowered to room temperature, and the reaction solution was concentrated under reduced pressure to obtain a solid crude product. The crude product was dissolved in 300 mL of toluene, and 150 mL of 10% K 2 CO 3 (mass fraction) washed twice, then washed with 150 mL of water, separated the organic phase, and concentrated under reduced pressure to obtain compound (2): 86.0 g, yield 96%, purity 98.1%; detection:
[0083] 1 H NMR (400MHz, CDCl 3 )δ9.49–8.48(m,2H),8.09(s,2H),7.77–7.58(m,2H),4.03(s,6H).
Embodiment 2
[0084] Preparation of Example 2 Compound (2)
[0085] Compound (1), namely 1,4 naphthalene dicarboxylic acid (50 g, 231 mmol) and methanol (350 mL) was added to a 1000 mL round-bottomed flask, and concentrated sulfuric acid (47.6 g, 485.7 mmol) was slowly added dropwise with stirring. After the dropwise addition was completed, The temperature was raised to 75°C, and the reaction was refluxed for 12h. After the reaction, it was lowered to room temperature, and the reaction solution was concentrated under reduced pressure to obtain a solid crude product. The crude product was dissolved in 400 mL of toluene, and 100 mL of 10% K 2 CO 3 (mass fraction) was washed twice, then washed with 150 mL of water, the organic phase was separated, and concentrated under reduced pressure to obtain compound (2): 53.0 g with a yield of 93.8% and a purity of 95%.
Embodiment 3
[0086] Preparation of Example 3 Compound (2)
[0087] Compound (1), namely 1,4 naphthalene dicarboxylic acid (10 g, 46.2 mmol) and methanol (70 mL) was added to a 250 mL round-bottomed flask, and concentrated hydrochloric acid (11.5 mL, 138.6 mmol) was slowly added dropwise with stirring. , the temperature was raised to 75°C, and the reaction was refluxed for 12h. After the reaction was completed, it was lowered to room temperature, and the reaction solution was concentrated under reduced pressure to obtain a solid crude product. The crude product was dissolved in 100 mL of ethyl acetate, and 50 mL of 10% K 2 CO 3 (mass fraction) washed twice, then washed with 50 mL of water, separated the organic phase, concentrated under reduced pressure to obtain compound (2): 18.3 g, yield 81.1%, purity 95.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com