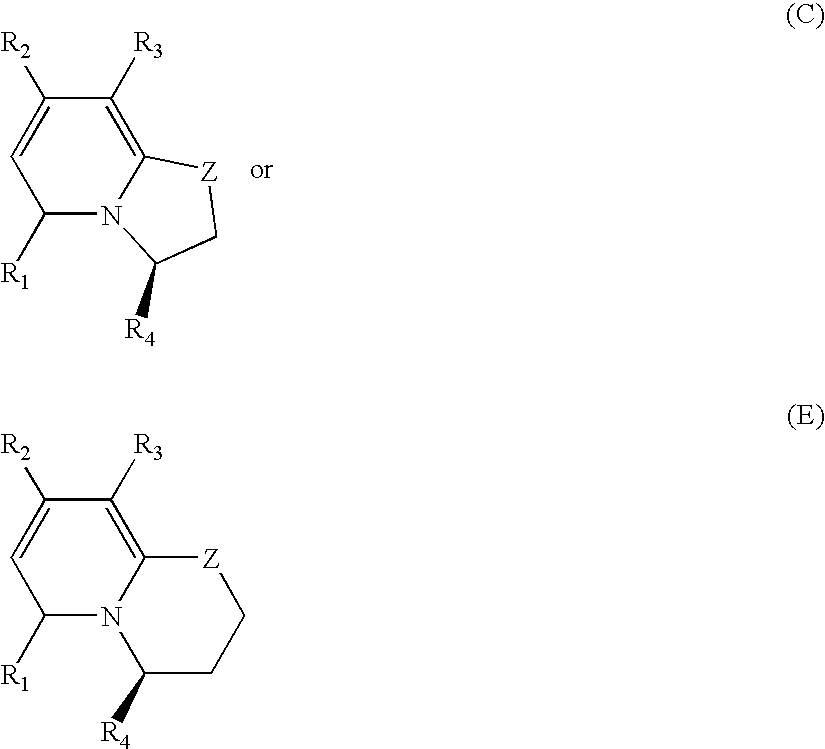
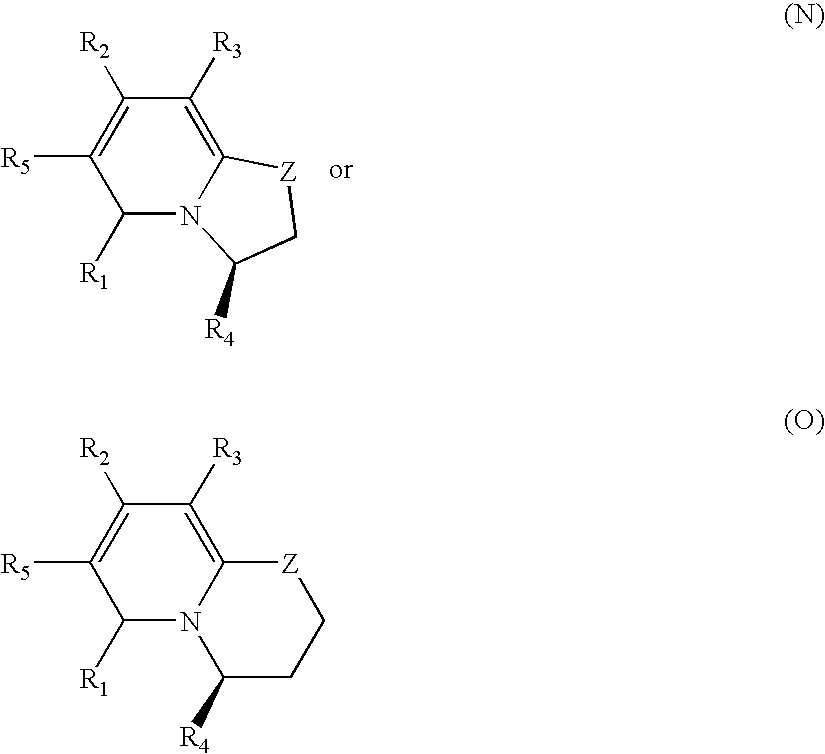
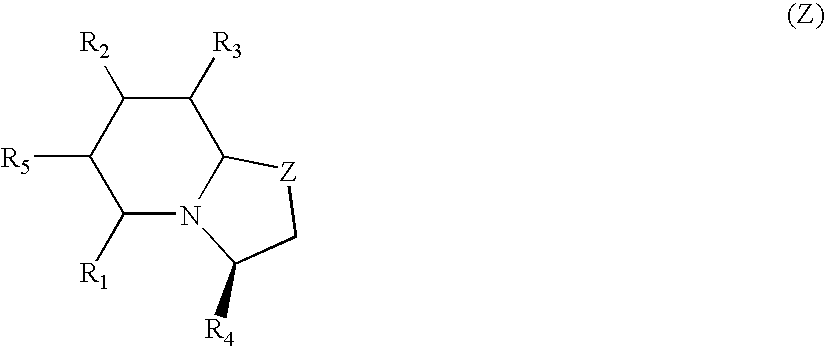
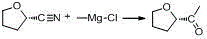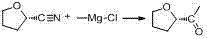Patents
Literature
61 results about "Meldrum's acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
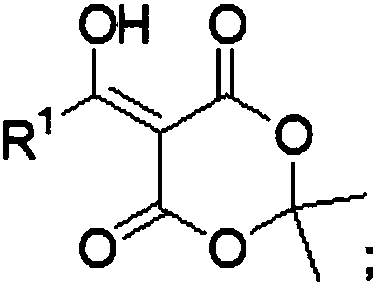
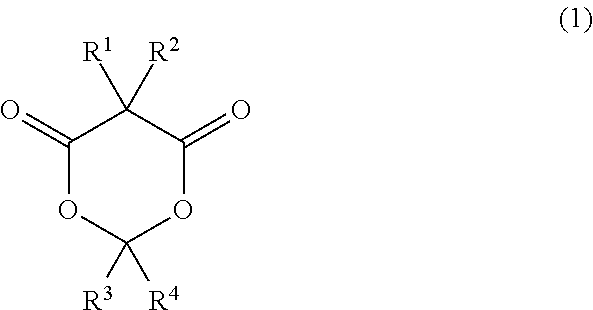
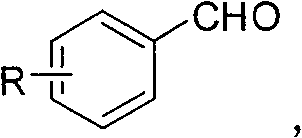
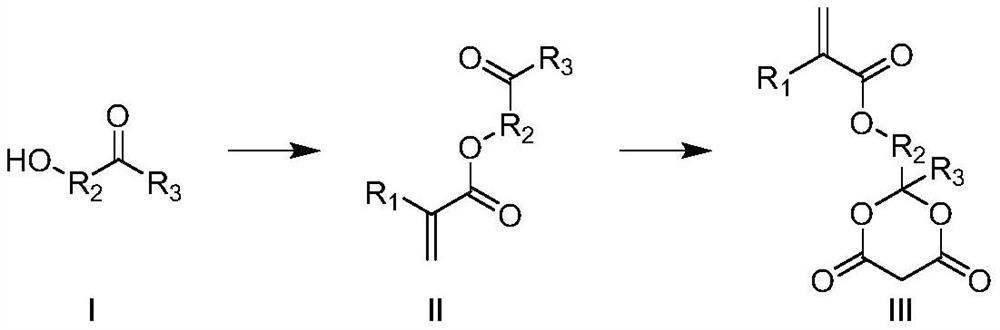
Meldrum's acid or 2,2-dimethyl-1,3-dioxane-4,6-dione is an organic compound with formula C6H8O4. Its molecule has a heterocyclic core with four carbon and two oxygen atoms; the formula can also be written as [–O–(C(CH3)2)–O–(C=O)–(CH2)–(C=O)–].
Coating solution for forming polyimide film, liquid crystal alignment agent, polyimide film, liquid crystal alignment film, and liquid crystal display element
ActiveCN103415583AImprove featuresHave a hardening effectCoatingsNon-linear opticsCrystallographyLiquid-crystal display
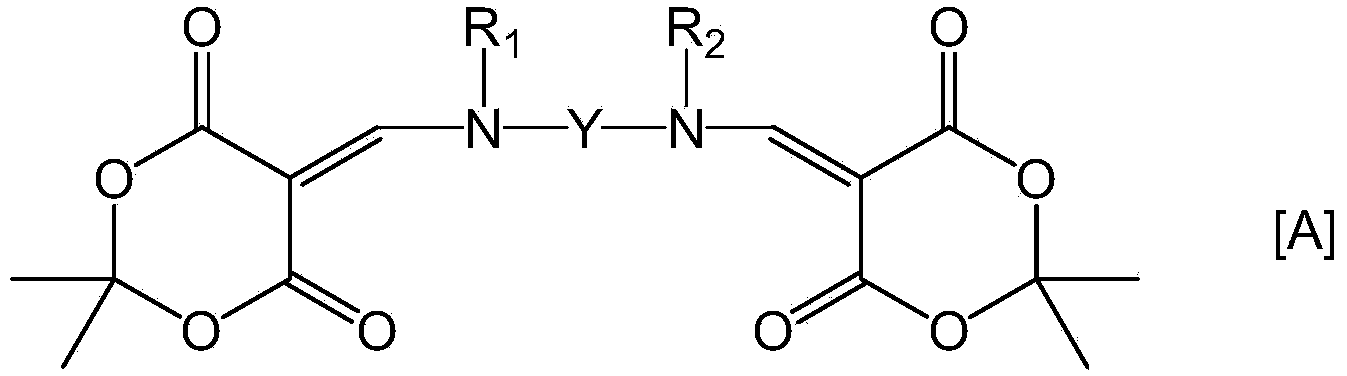
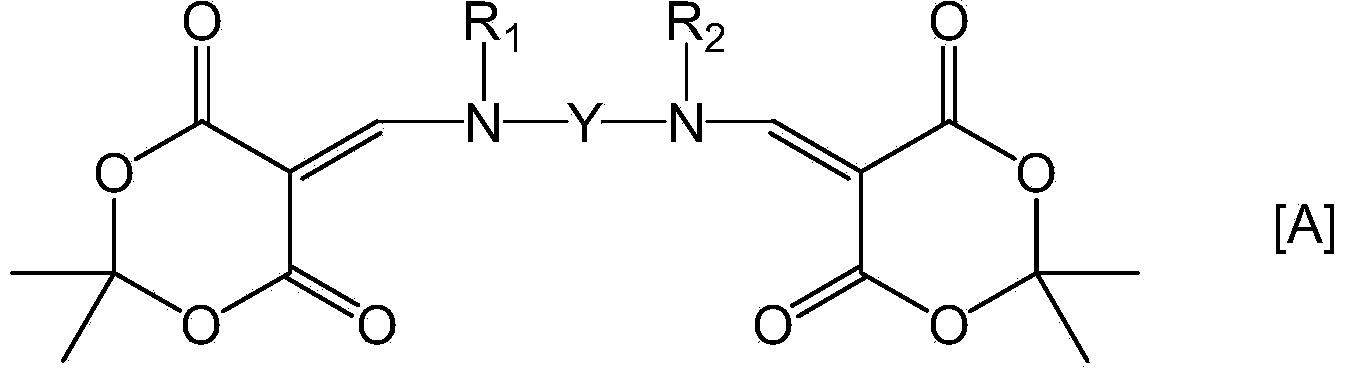
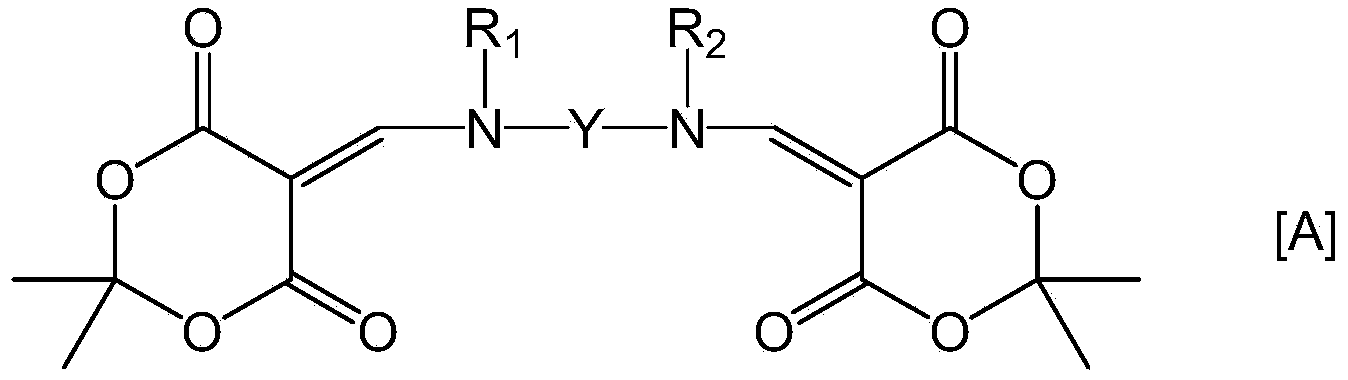
Provided is a coating solution for forming a polyimide film, said coating solution comprising: a polymer of a polyimide precursor obtained by polymerizing a tetracarbonate component and a diamine component, and / or a polyimide formed by the imidization of the polyimide precursor; and a bifunctional compound represented by formula [A] and having a Meldrum's acid structure introduced into each of two amino groups of a diamine compound. Also provided are: a liquid crystal alignment agent comprising said coating solution; a polyimide film obtained by firing a substrate coated using said coating solution; a polyimide film comprising a polyimide in which the polymer is crosslinked using the bifunctional compound represented by formula [A]; a liquid crystal alignment film comprising the polyimide film; and a liquid crystal display element equipped with said liquid crystal alignment film. (In the formula, Y, R1 and R2 are groups specified in claims 1 and 4.)
Owner:NISSAN CHEM IND LTD
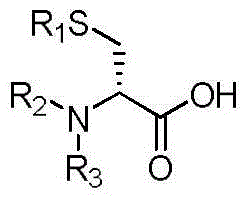
Chiral synthesis method for chiral beta-amino acid and synthesis method for medicinal intermediate
ActiveCN107501112AEasy to synthesizeLow costCarbamic acid derivatives preparationOrganic compound preparationSynthesis methodsHydrolysis
The invention relates to a chiral synthesis method for chiral beta-amino acid. The chiral synthesis method comprises the following steps: reacting a compound shown in formula (II) with an acylation reagent to prepare anhydride intermediate reaction liquid under the action of alkali; adding Meldrum's acid into the anhydride intermediate reaction liquid, and performing reaction to generate a compound shown in formula (III); reacting the compound shown in formula (III) with a compound shown in formula (IV) to generate a compound shown in formula (V); reducing the compound shown in formula (V) to generate a compound shown in formula (VI); performing acidic hydrolysis on the compound shown in formula (VI) to generate a compound shown in formula (I), i.e., the chiral beta-amino acid. The chiral synthesis method has the advantages of convenience for synthesis, low cost and simple process, and compared with a disclosed preparation method, is more suitable for industrial production.
Owner:苏州爱玛特生物科技有限公司
Adhesive resin composition, laminate, and self-stripping method
InactiveUS20140322474A1Promote decompositionEasily contaminatedOrganic chemistryNon-macromolecular adhesive additivesPolymer scienceAcid derivative
An adhesive resin composition of the present invention includes an expandable sticky polymer having a structure derived from a Meldrum's acid derivative, or a Meldrum's acid derivative represented by the following general formula (1) and an adhesive resin.
Owner:MITSUI CHEM TOHCELLO INC
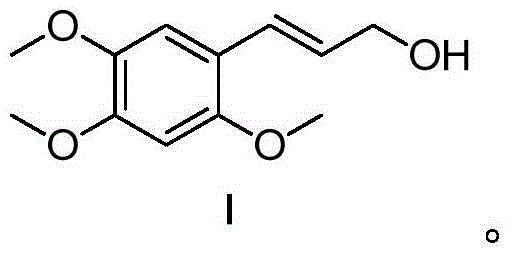
(E)-3-(2,4,5-trimethoxy-phenyl)-prop-2-en-1-ol , and preparation method and application thereof
ActiveCN104529724AGood anticonvulsant effectCardiovascular protectionNervous disorderOrganic chemistryConvulsionMalonate
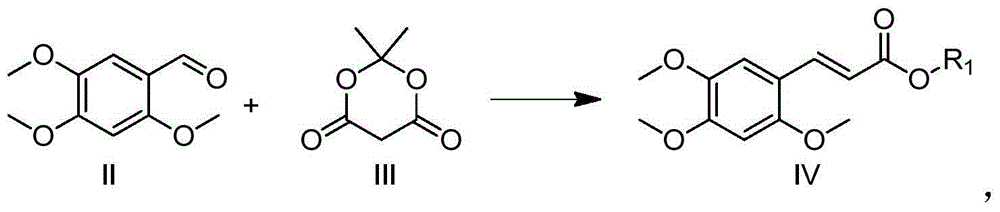
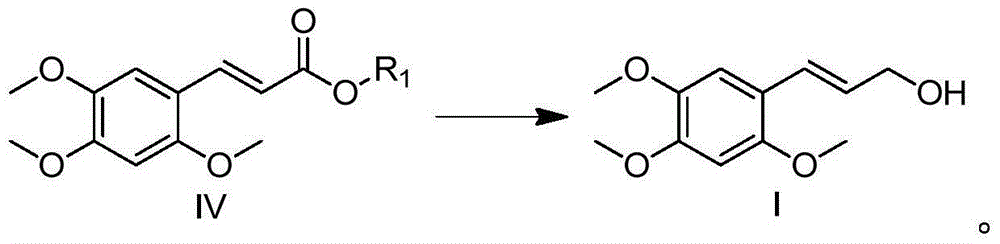
The invention relates to (E)-3-(2,4,5-trimethoxy-phenyl)-prop-2-en-1-ol, and a preparation method and application thereof. Related (E)-3-(2,4,5-trimethoxy-phenyl)-prop-2-en-1-ol has the structural formula I shown in the specification. The related preparation method comprises: performing decarboxylation reaction on 2,4,5-trimethoxybenzaldehyde, isopropylidene malonate (meldrum's acid) and a fatty alcohol under catalytic effect of pyridine and piperidine, so as to obtain (E)-2,4,5-trimethoxycinnamate, and processing by using a reducing agent, so as to obtain (E)-3-(2,4,5-trimethoxy-phenyl)-prop-2-en-1-ol. The preparation method is simple and relatively high in yield. The related application comprises that (E)-3-(2,4,5-trimethoxy-phenyl)-prop-2-en-1-ol is applied to prepare medicines for calming, tranquilizing, resisting senile dementia, resisting convulsion, resisting epilepsy or protecting heart and cerebral vessels.
Owner:NORTHWEST UNIV +1
Preparation method of anti-tumor medicine chlorambucil
InactiveCN107628962ALow equipment requirementsReduce manufacturing costOrganic compound preparationAmino-carboxyl compound preparationAbnormal tissue growthBenzaldehyde
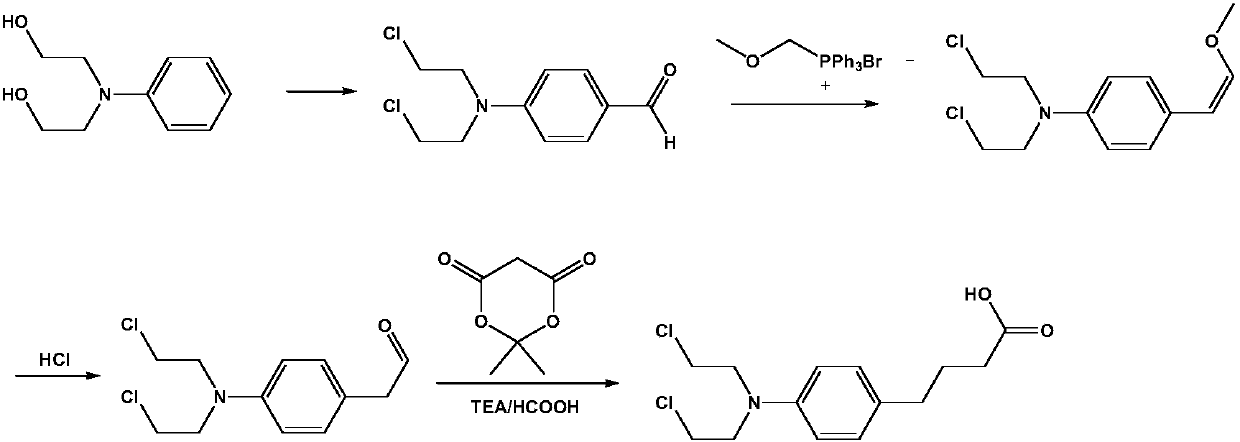
The invention belongs to the field of compound preparation, and specifically discloses a preparation method of an anti-tumor medicine chlorambucil. The preparation method comprises the steps of performing a Vilsmeier reaction on a raw material N,N-dihydroxyethylaniline and phosphorus oxychloride and DMF so as to prepare 4-[bi(2-chloroethyl)amino]benzaldehyde, then performing a witting reaction on4-[bi(2-chloroethyl)amino]benzaldehyde and methoxymethyl triphenylphosphonium chloride so as to prepare 4-[bi(2-chloroethyl)amino]-BETA-methoxystyrene, reacting under an acid condition so as to obtain4-[bi(2-chloroethyl)amino]phenylacetaldehyde, and finally, reacting with Meldrum's acid in triethylamine and formic acid systems so as to prepare a target product. The raw material N,N-dihydroxyethylaniline is cheap, has a wide source and is easy to obtain; the whole reaction process has high yield, production conditions are mild, the steps are short, and post treatment and purification are easyto operate, so that the preparation method is applicable to commercial large scale production, and meets the rapidly growing market demands.
Owner:TIANJIN DERCHEMIST SCI TECH
Method for synthesizing 4-hydroxyl coumarin and derivant thereof
InactiveCN101220016ALow temperature requirementLess investmentOrganic chemistryChemical recyclingPhosphoric acidYield ratio
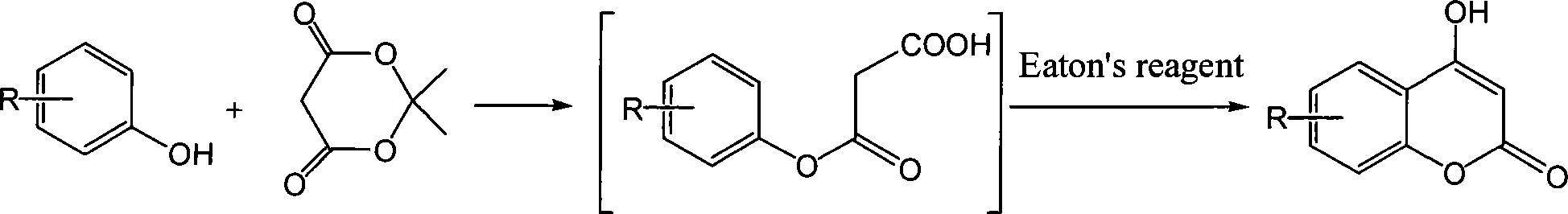
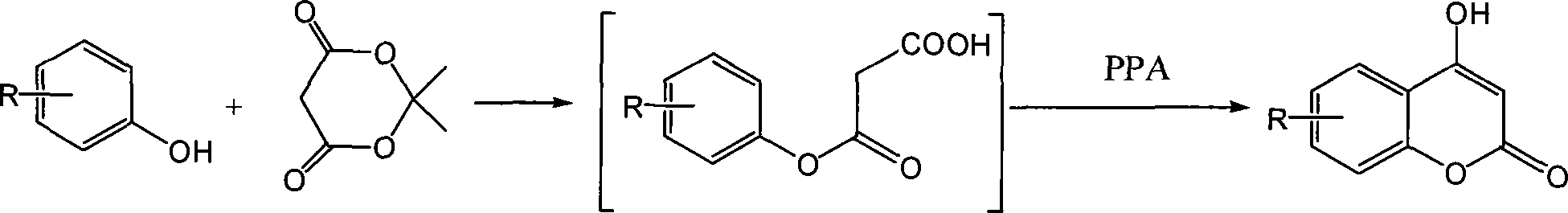
A specific technique of a method of synthesizing 4-hydroxycoumarin and derivatives of 4-hydroxycoumarin is as follows: taking a phenol or the derivatives of the phenol and an Meldrum's acid as the raw materials, using a thin layer method to monitor and then decompressing to remove an acetone after complete reaction; with no necessity of separating an intermediate product, directly carrying out intermolecular dehydration with an ito agent or a polyphosphoric and ring-closing reaction and finally adding water to precipitate a sedimentation, filtrating and then recrystallizing to get a final product---4-hydroxycoumarin and the derivatives of 4-hydroxycoumarin. The proportion of the phenol, the derivatives of the phenol, the Meldrum's acid, the ito agent or the polyphosphoric is 1.0mmol:1.0mmol:1-3ml or 1 to 2g. The temperature of intermolecular ring-closing reaction when adding the ito agent is 60 DEG C to 70 DEG C; the reaction time is 1 to 5 hours. The temperature of intermolecular ring-closing reaction when adding the polyphosphoric is 110 DEG C to 120DEG C; the reaction time is 3 to 7 hours. The method has the advantages of easy raw material obtaining, mild reaction condition, simple operation condition and less pollution. Furthermore, the yield varies from medium to excellence; no catalyst is needed; the method also assures a high yield ratio.
Owner:BOHAI UNIV
Pyridinones to treat and prevent bacterial infections
Novel pyridinones and their derivatives which are effective in treating or preventing Gram-negative bacterial infections are provided. The pyridinones are stable and easily derivatized; the methods by which these derivatizations occur is described. Two regioselective and functional group tolerant methods for the synthesis of the novel pyridinones are also provided. One such synthetic method involves reacting an imine and a Meldrum's acid derivative in solution. The other synthetic method is a solid phase synthesis of the pyridinones in which an imine is prepared bound to a solid support and a Meldrum's acid derivative is reacted with the imine. Novel imine intermediates useful in the solid phase and solution methods of synthesizing the pyridionones are also described.
Owner:WASHINGTON UNIV IN SAINT LOUIS
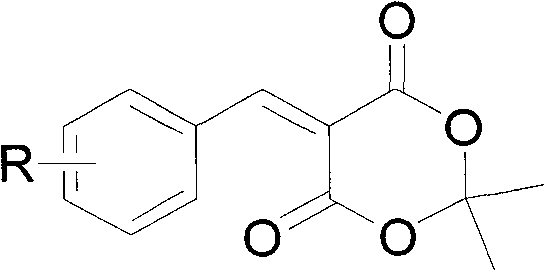
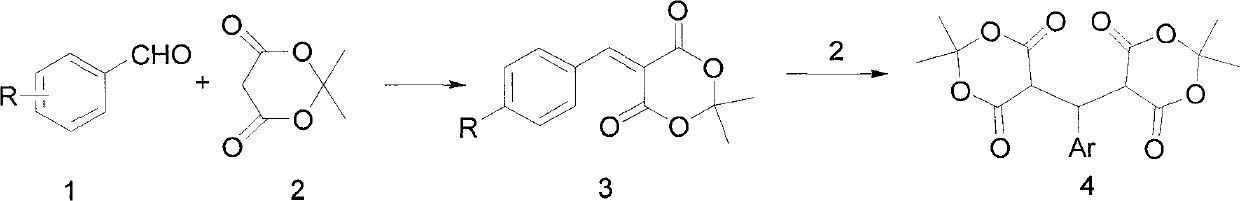
Preparation method of 5-( arylmethylene) meldrum's acid
The invention relates to a preparation method of 5-(arylmethylene) meldrum's acid, which comprises the following steps of: a, dissolving aromatic aldehyde and aniline according to the mol rate of 1.0:(1.0-1.2) into water, wherein the aromatic aldehyde has a structural formula described in the specification, R is H, X (halogen), alkyl (such as CH3, C2H5), alkoxy (such as OCH3, OCH2CH3), OH, NO2 and CN; and b, respectively dissolving arylmethylene aniline obtained from the step a and meldrum's acid into an alcohol solution according to the mol ratio of (1.1-1.2):1.0 to obtain respective saturated solutions, then adding the alcohol saturated solution of the arylmethylene aniline into the alcohol saturated solution of the meldrum's acid, stirring for reacting for 3-4min, filtering after the reaction is ended, washing filter residue with water, drying and then recrystallizing to obtain the 5-(arylmethylene) meldrum's acid. The preparation method has the advantages of environment protection because of using water and alcohol as solvents, mild reaction condition, convenient posttreatment and higher yield of products.
Owner:SHANGHAI UNIV
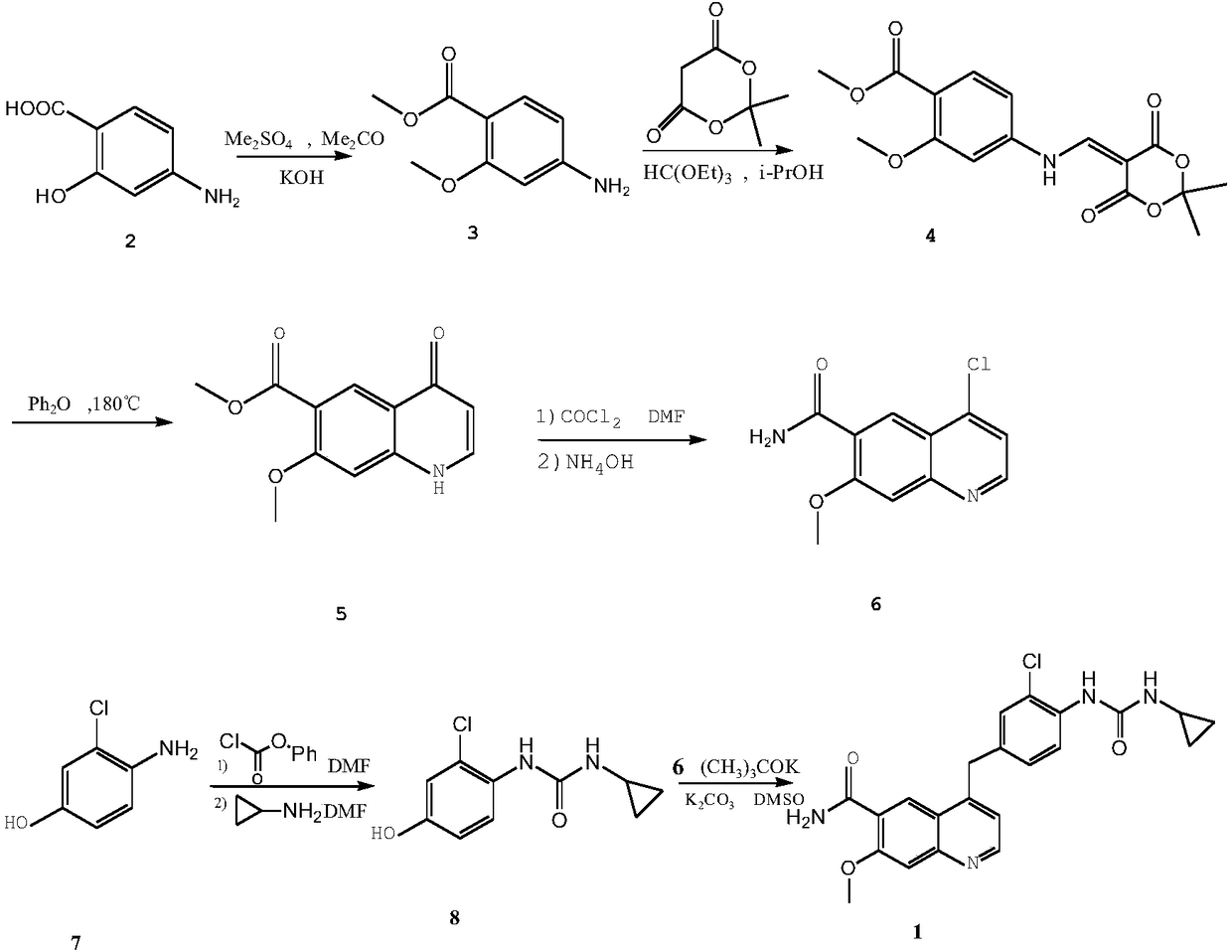
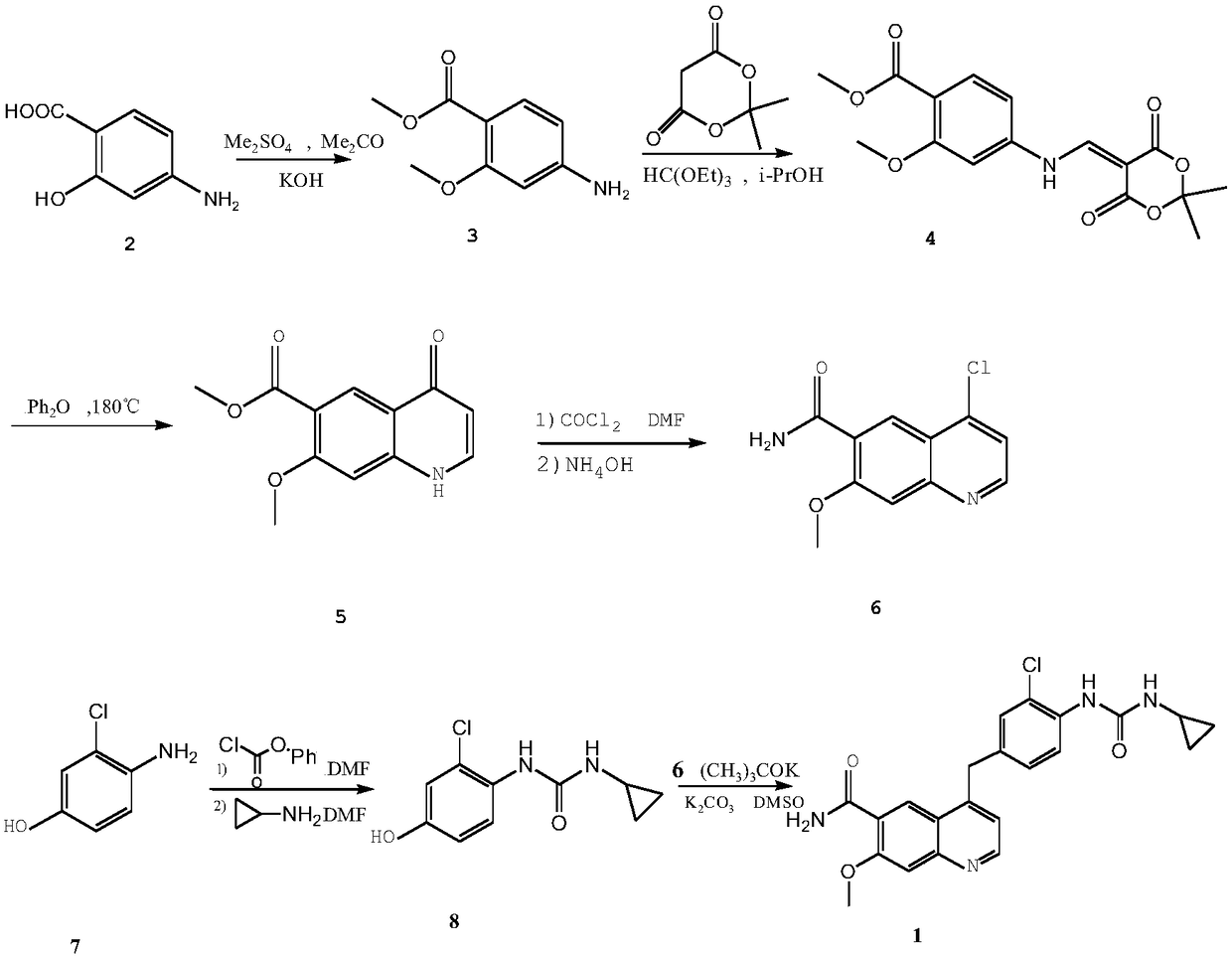
Method for synthesizing lenvatinib
The invention belongs to the field of chemical pharmacy, and specifically relates to a method for synthesizing lenvatinib. The method comprises the following steps: step 1, taking 4-aminosalicylic acid as a raw material, and preparing 4-chloro-7-methoxyquinoline-6-formamide through methylation, condensation with meldrum's acid, high-temperature cyclization, chlorination and ammoniation; step 2, taking 3-chloro-4-aminophenol as a raw material, and reacting with phenyl chloroformate and cyclopropylamine to obtain 1-(2-chloro-4-hydroxy phenyl)-3-cyclopropyl urea; and step 3, enabling the 4-chloro-7-methoxyquinoline-6-formamide prepared in step 1 to react with the 1-(2-chloro-4-hydroxy phenyl)-3-cyclopropyl urea prepared in step 2 under action of potassium tert-butoxide to obtain the lenvatinib. The invention provides a brand-new route for synthesising the lenvatinib. The used reagent is cheap and is easily available, is simple in operation, has a yield higher than that of other methods, and is easy for industrial production.
Owner:南京天越星生物技术有限公司
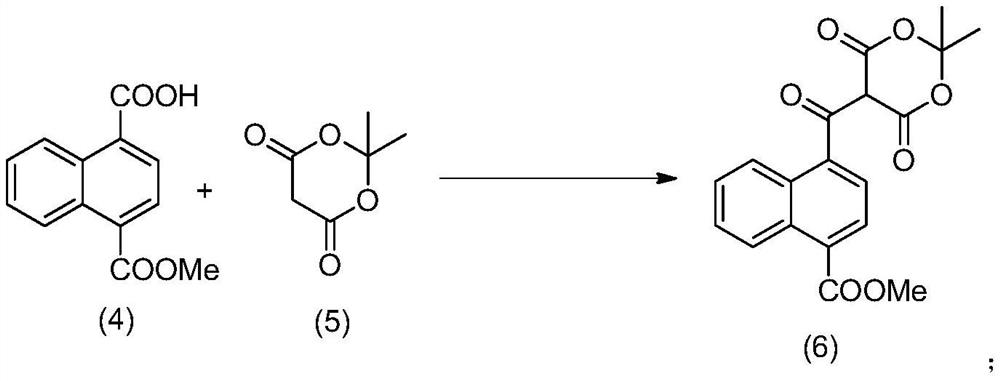
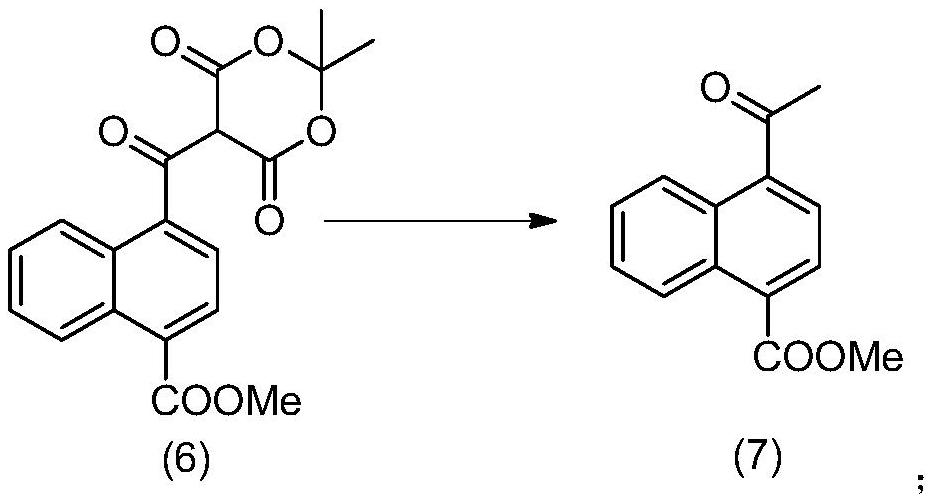
Method for preparing 4-acetyl-1-naphthoic acid
PendingCN113354530AOrganic compound preparationCarboxylic acid esters preparationHydrolysisPharmaceutical technology
The invention relates to a method for preparing 4-acetyl-1-naphthoic acid, and belongs to the technical field of pharmacy. According to the method provided by the invention, 1, 4-naphthalic acid which is low in price and easy to obtain is taken as a raw material, and 4-acetyl-1-naphthoic acid is prepared by adding Meldrum's acid, performing decarboxylation to formylate carboxyl, and finally performing hydrolysis. According to the method provided by the invention, a high-purity product can be obtained, an inflammable metal reagent methyl zinc is not used in the process, the reaction condition is mild, the operation is simple, and the method is suitable for industrial large-scale production.
Owner:东莞市东阳光动物保健药品有限公司
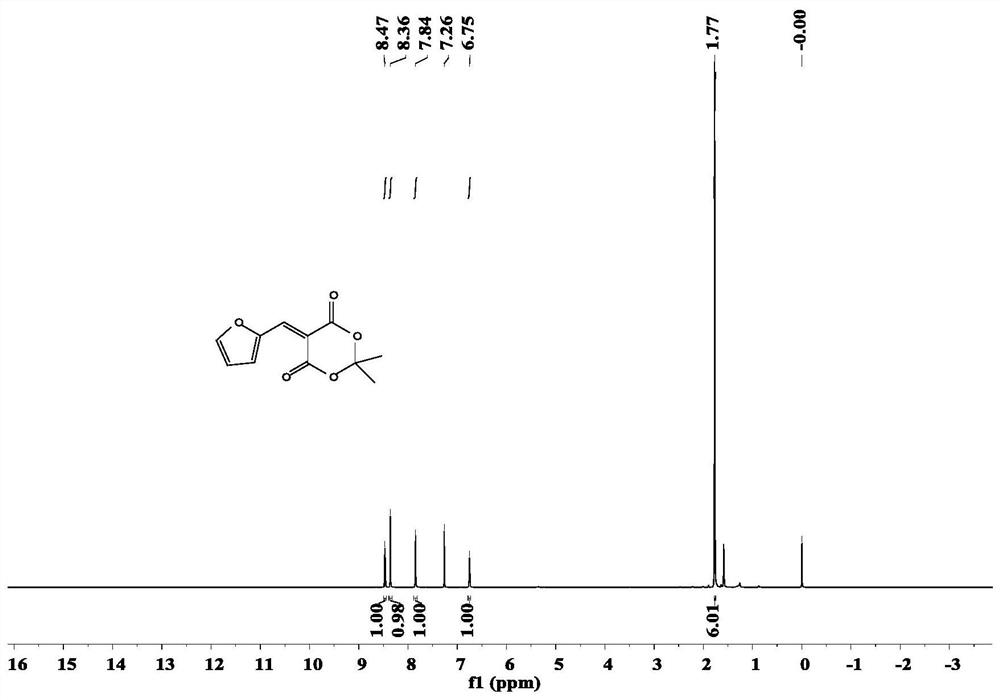
Stenhouse donor-receptor adduct of Meldrum's acid-activated furan and 3-pyridylethylamine, and synthesis method thereof
The invention discloses a Stenhouse donor-receptor adduct (DASA) based on Meldrum's acid activated furan and 3-pyridylethylamine, and a synthesis method of the Stenhouse donor-receptor adduct. The method specifically comprises the following steps: mixing 2-furan formaldehyde and cycloisopropyl malonate to react to synthesize an intermediate, and reacting the intermediate with 3-pyridylethylamine to generate a DASA compound. According to the method, the organic solvent with low toxicity is used for carrying out addition reaction under certain conditions to obtain the DASA compound with high yield and high content, and a catalyst is not needed, so that the production cost is effectively reduced; and the photophysical property test shows that the compound has different performances in different solvents, which indicates that the compound has a wide application range, can satisfy different demands in different fields, and fully exerts the excellent performances of the compound.
Owner:NANJING UNIV OF SCI & TECH
Tetrahydropyridone 1,3-diazacyclo cis-neonicotine compounds as well as preparation method and applications thereof
The invention belongs to a pesticide and discloses tetrahydropyridone 1,3-diazacyclo cis-neonicotine compounds shown in a general formula (I) as well as a preparation method and applications thereof. The preparation method comprises the following steps: using ethanol as solvent, and adding neonicotine compounds, aldehyde and Meldrum's acid; and adding piperidine used as a catalyst, and refluxing to react. In the method, a tetrahydropyridone structure is introduced in the neonicotine compounds for the first time. The tetrahydropyridone 1,3-diazacyclo cis-neonicotine compounds have the following advantages: the preparation process is simple, and the yield is high; the preparation process has no pollution; and the compounds have good insecticidal effects and are safe for human and livestock.
Owner:SHANGHAI NORMAL UNIVERSITY
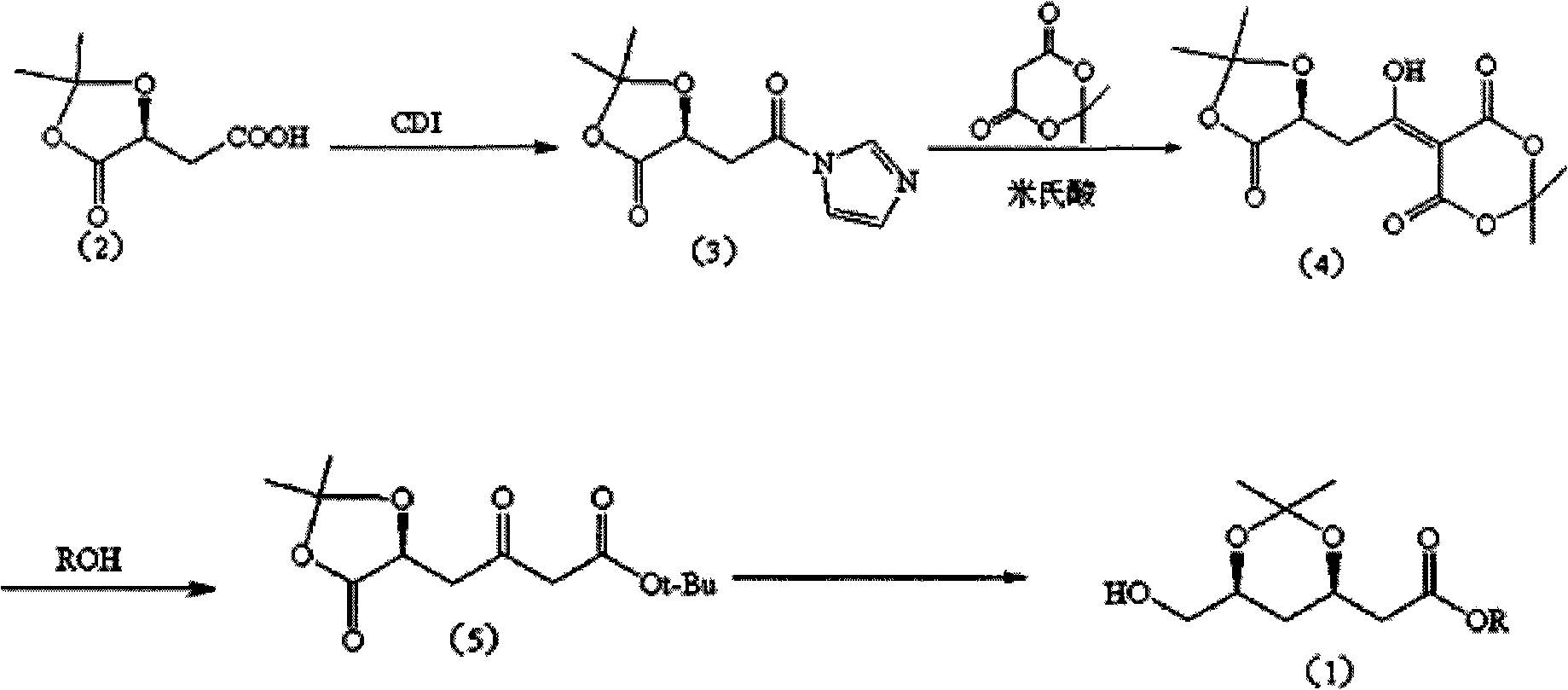
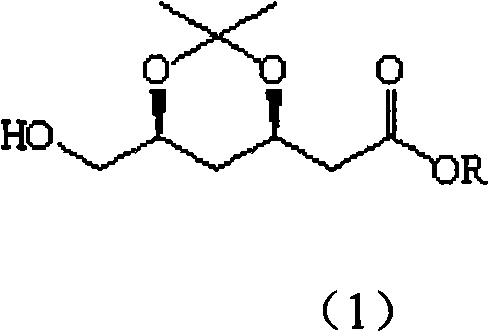
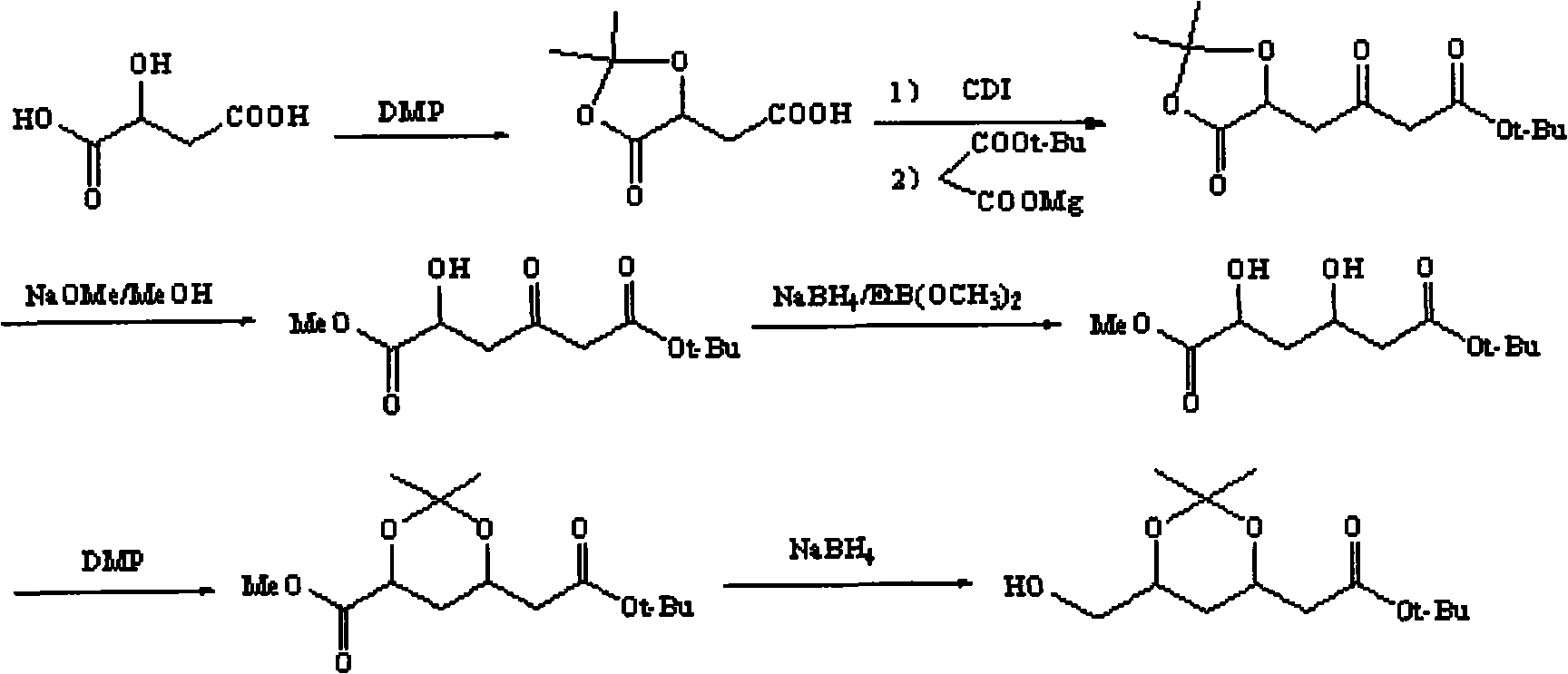
Method for preparing (3R, 5S)-3, 5-O-isopropylidene-3, 5, 6-trihydroxy caproic acid hexylic acid derivative
ActiveCN101538261AEasy to storeReduce usageOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSolventTert butyl
The invention discloses a method for preparing (3R, 5S)-3, 5-O-isopropylidene-3, 5, 6-trihydroxy caproic acid hexylic acid derivative, including the following steps: (1) L-malic acid protected hydroxy compound and carbonyl diimidazole (CD I) react in solvent to obtain (S)-2,2- dimethyl-5-oxo-1, 3-dioxa-4-acetyl imidazole; (2) the (S)-2,2- dimethyl-5-oxo-1, 3-dioxa-4-acetyl imidazole and Meldrum's acid react in the solvent under the existence of basic catalyst to prepare (S)-2-(4'-oxo-3', 4'-O-isopropylidene-3', 4'-dihydroxy-1'-hydroxy-alkene)-malonic acid cyclic isopropylidene ester; (3) the (S)-2-(4'-oxo-3',4'-O-isopropylidene-3',4'-dihydroxy-1'-hydroxy-alkene)-malonic acid cyclic isopropylidene ester reacts with methanol to prepare (S)-3, 6-dioxo-5, 6-isopropylidene-5, 6-dihydroxy tert-butyl ester hexanoate; and a target compound can be obtained after further reaction. The method has the advantages of easy acquisition of materials, moderate reaction condition, less material consumption, high product quality and yield and easy implementation of industrialization.
Owner:SICHUAN INDAL INST OF ANTIBIOTICS CHINA NAT PHARMA GROUP CORP +1
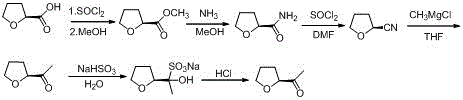
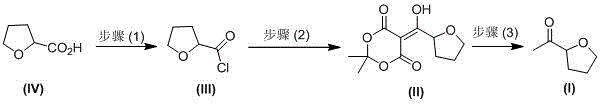
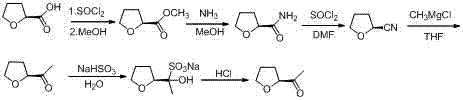
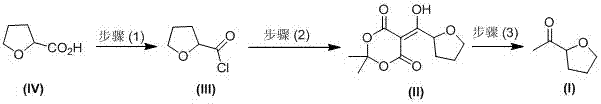
Industrial preparation method of acetyl tetrahydrofuran with high optical purity
The invention discloses an industrial preparation method of acetyl tetrahydrofuran with a high optical purity, and belongs to the field of chemical synthesis. According to the preparation method, tetrahydrofuroic acid is taken as the raw material and then is chlorinated to obtain tetrahydrofuran carbonyl chloride, tetrahydrofuran carbonyl chloride and Meldrum's acid carry out condensation reactions, and reaction product is hydrolyzed to obtain the target compound namely acetyl tetrahydrofuran. The preparation method has the advantages that the raw material cost is low, the preparation method does not need any Grignard reagent, the product property is stable, the purity can reach 98% or more, the optical purity can reach 99% or more, and the yield can reach 70% or more. The method has applied to industrial production. The product quality is stable. The reaction conditions are mild. The operation is safe and reliable. Dichloromethane can be recycled. The technology has the advantages of good repeatability and low preparation cost, and is a reliable industrial production method of acetyl tetrahydrofuran with a high optical purity.
Owner:CHENGDU LIKAI CHIRAL TECH
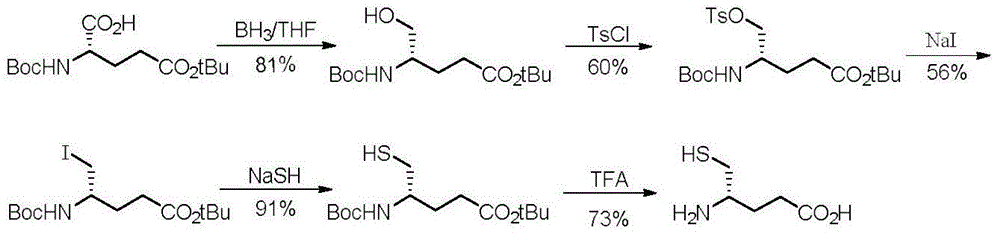
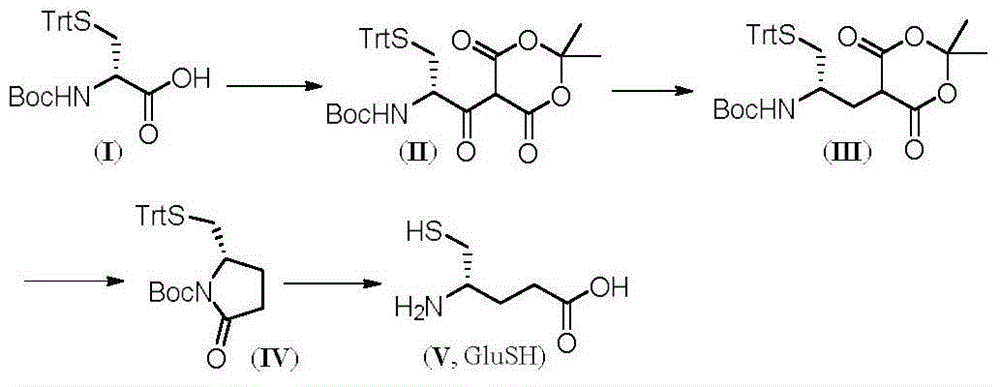
(S)-4-amino-5-mercaptopentanoic acid preparation method
InactiveCN106187844AShort reaction stepsEasy to purifyThiol preparationBulk chemical productionMeldrum's acidKetone
The present invention belongs to the technical field of medicine, and relates to a preparation method of (S)-4-amino-5-mercaptopentanoic acid having potential medical application value. According to the method of the present invention, amino-protected and mercapto-protected D-cysteine is adopted as a raw material and is subjected to condensation with Meldrum's acid to generate beta-ketone ester, the ketone carbonyl is subjected to reducing elimination, lactam is formed through decarboxylation, the protection groups on mercapto and amino are removed, and the lactam ring is opened to prepare the (S)-4-amino-5-mercaptopentanoic acid. According to the present invention, the method has following advantages that reaction steps are short, wherein only the four steps are required; the purification is easy, wherein only the simple washing operation is required; and the yield is high, wherein the total yield of the four steps is 76.0%.
Owner:FUDAN UNIV
Synthetic method of oat alkaloids
InactiveCN108456149AOrganic compound preparationCarboxylic acid amides preparationChemical synthesisBenzaldehyde
The invention provides a synthetic method of oat alkaloid and studies a chemical synthesis process of oat alkaloids A, B and C. The oat alkaloids A, B and C are synthesized by taking 2-amino-5-hydroxybenzoic acid, meldrum's acid, 4-hydroxybenzaldehyde, 4-hydroxyl-3-methoxybenzaldehyde and 3,4-dihydroxy benzaldehyde as raw materials and using a Knoevenagel condensation reaction. Analysis means suchas infrared spectroscopy, nuclear magnetic resonance spectroscopy, mass spectroscopy and elemental analysis are applied to perform structural characterization on a compound, so that the feasibility of a process route of synergizing the oat alkaloids is demonstrated, and the synthetic process conditions are optimized; the synthetic method has important research significance for further developmentof application of oat alkaloids with multiple bioactive functions such as oxidation resistance. The development of the chemical synthesis technology has a very broad application prospect.
Owner:YICHUN UNIVERSITY
Preparation method of hydroxychloroquine
ActiveCN111635358AMeet high purity quality requirementsAvoid generatingOrganic chemistryBulk chemical productionBenzoic acidHydroxychloroquine
The invention belongs to the technical field of medicine and chemical engineering, and particularly relates to a hydroxychloroquine preparation method. The method comprises: carrying out a condensation reaction on a quinoline intermediate 7-chloro-4-hydroxyquinoline sulfonate and a hydroxychloroquine side chain in a eutectic solvent to obtain a target product, wherein the preparation method of thequinoline intermediate 7-chloro-4-hydroxyquinoline sulfonate comprises the following steps: (1) by taking 4-chloro-2-nitrobenzoic acid as a raw material, carrying out a chlorination reaction to prepare acyl chloride, condensing the acyl chloride with Meldrum's acid, and hydrolyzing to obtain 4-chloro-2-nitroacetophenone; and (2) carrying out condensation reaction, nitro reduction cyclization andhydroxyl protection reaction on the 4-chloro-2-nitroacetophenone and N,N-dimethylformamide methylal to obtain the quinoline intermediate 7-chloro-4-hydroxyquinoline sulfonate. The method has the advantages of easily available raw materials, mild reaction conditions, difficulty in side reaction, avoidance of high-temperature production conditions, reduction of risks, good intermediate stability, high yield and good purity of the obtained hydroxychloroquine, and facilitation of large-scale production.
Owner:北京成宇化工有限公司
Preparation method for coumarin-3-carboxylic ester derivative
The invention discloses a preparation method for a coumarin-3-carboxylic ester derivative shown as the formula (I) or the formula (II) in the specification. The method comprises the step that under the existence of FeCl3 being used as a catalyst, salicylaldehyde derivative shown as the formula (III) in the specification and 2-hydroxy-1-naphthaldehyde shown as the formula (IV) in the specification are in contact reaction with alcohol and meldrum's acid which have a general formula, namely R2OH. Through the fact that the salicylaldehyde derivative, the 2-hydroxy-1-naphthaldehyde, the alcohol or the meldrum's acid is catalyzed by FeCl3 in multi-component contact reaction under a relatively temperate condition, the coumarin-3-carboxylic ester derivative with relatively high productivity is synthesized in relatively short reaction time, and the method is simple in step, efficient and environment-friendly, and completely meets the concept of green chemistry.
Owner:ANHUI NORMAL UNIV
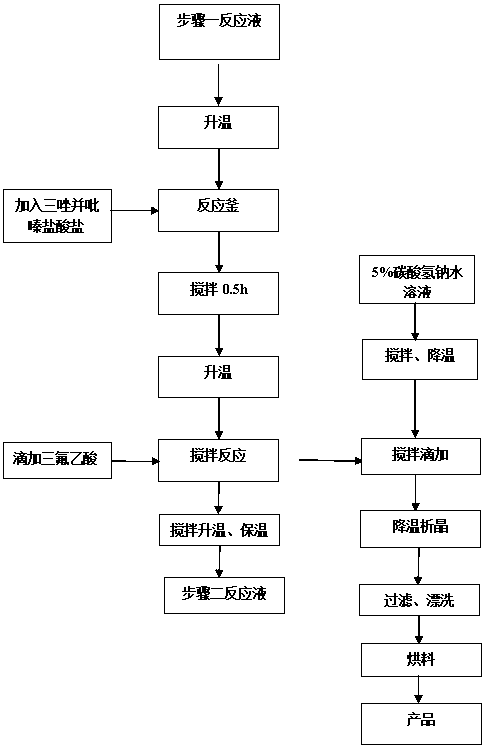
Synthesis method of sitagliptin phosphate intermediate
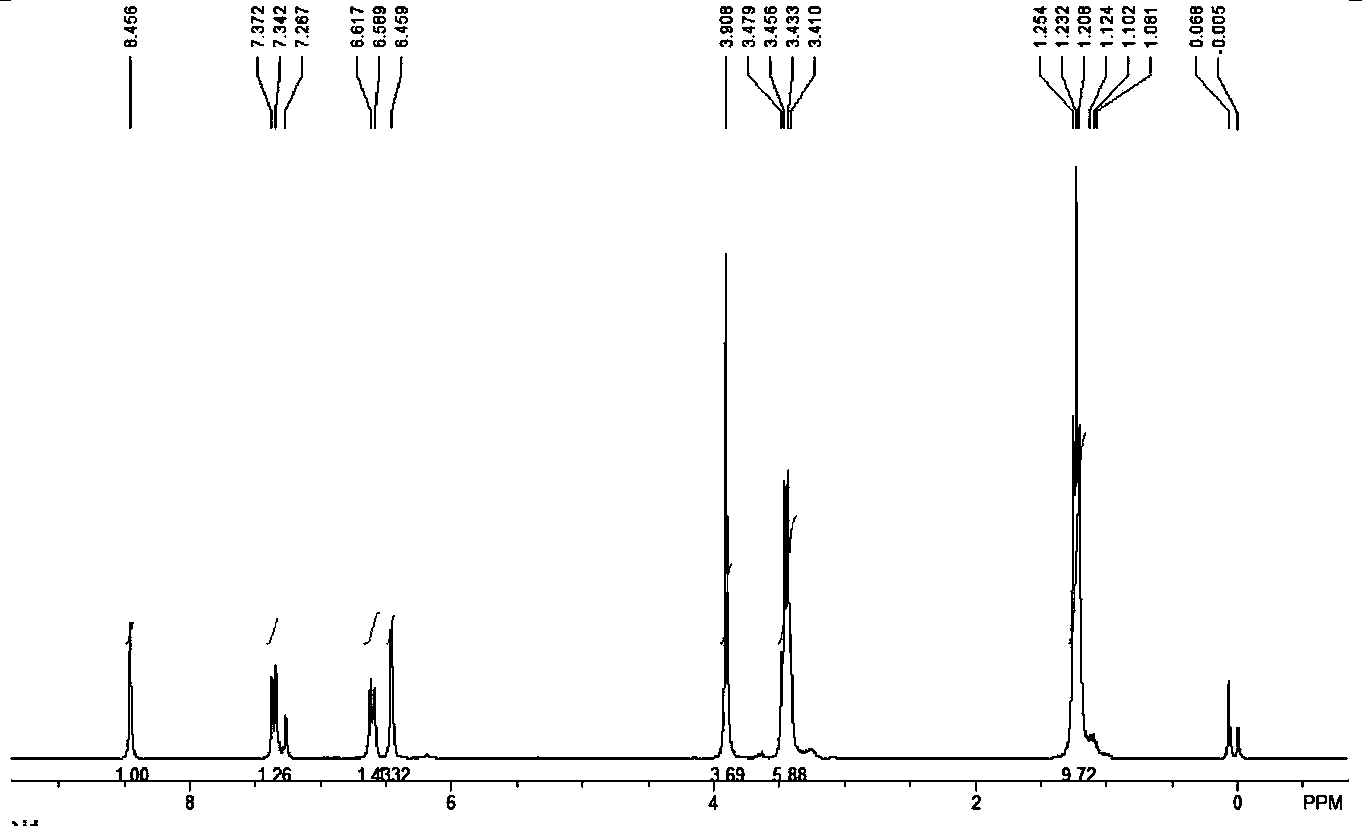
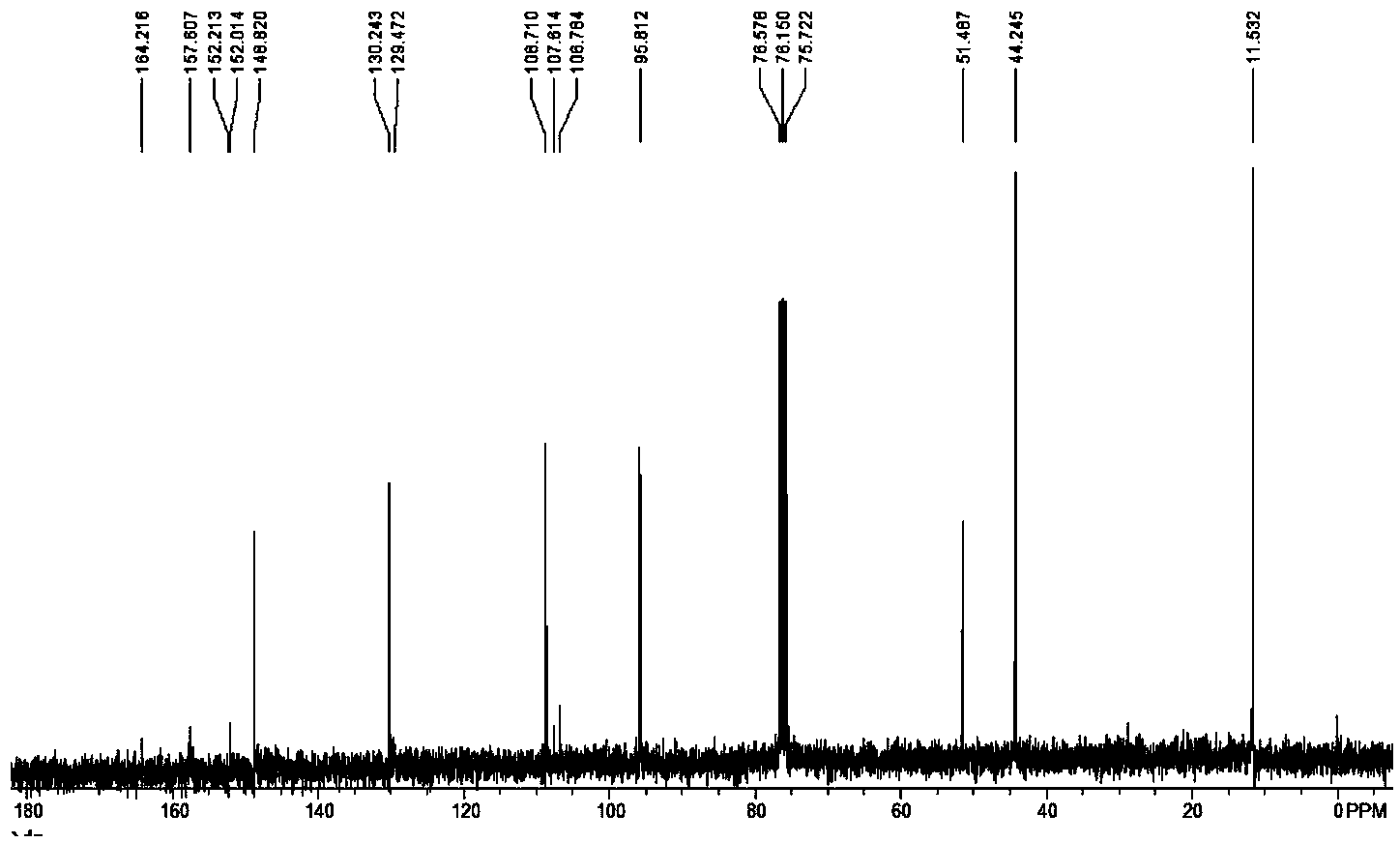
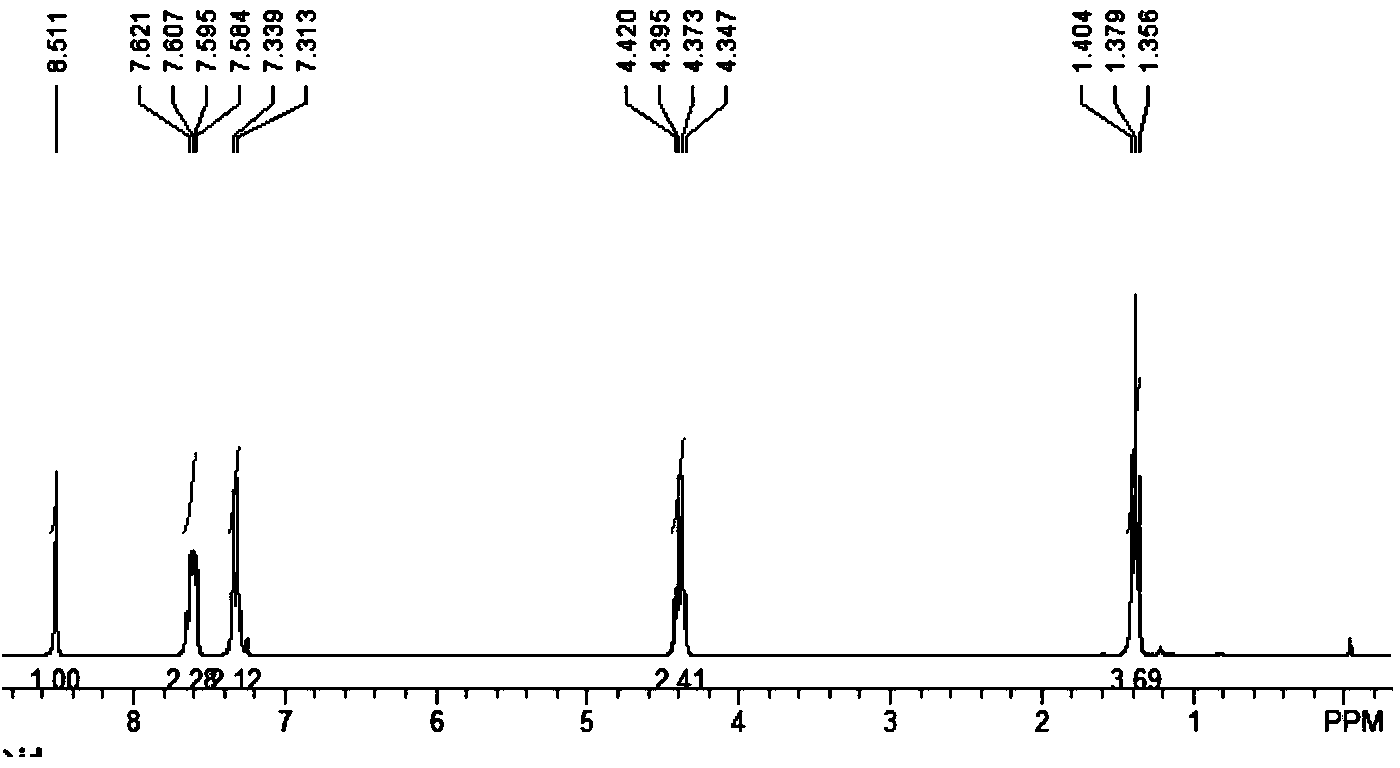
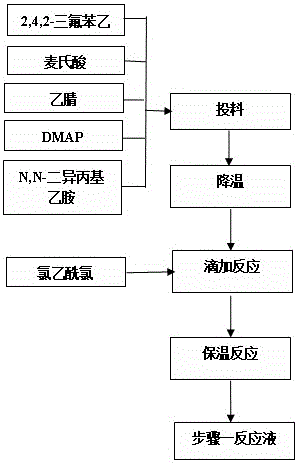
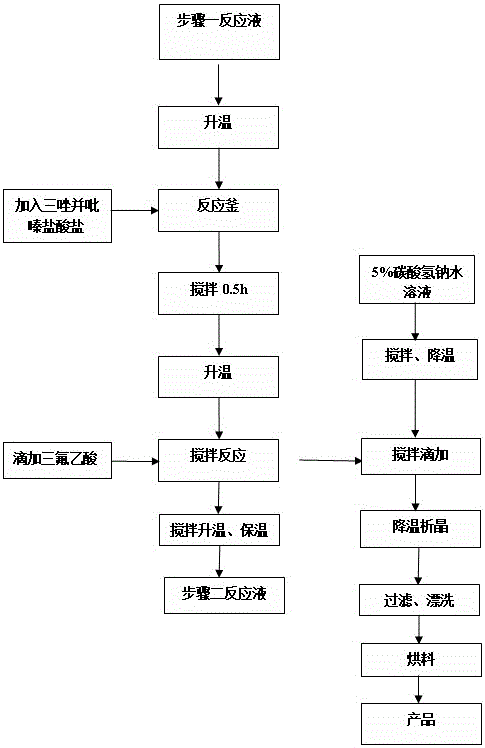
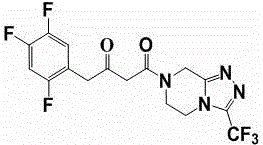
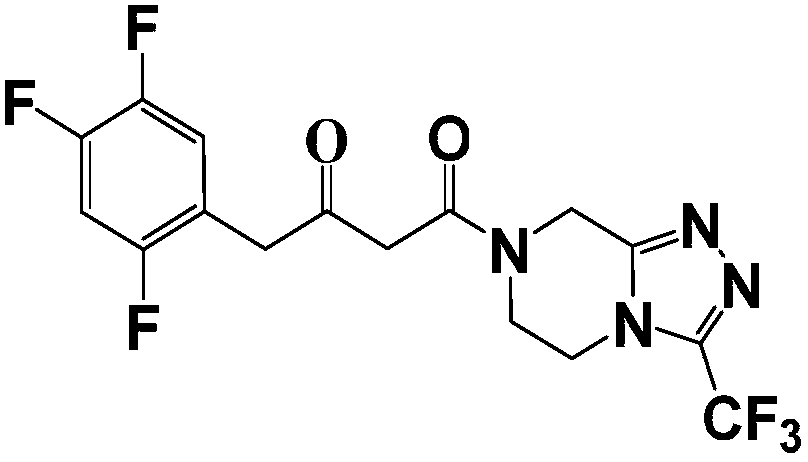
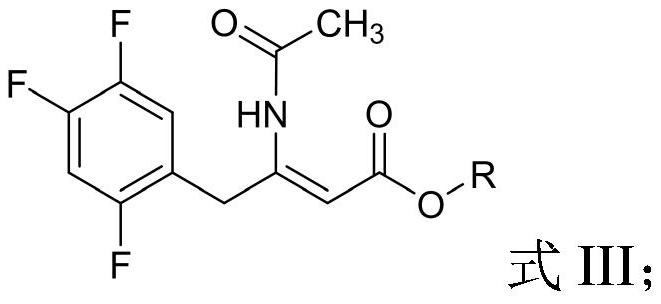
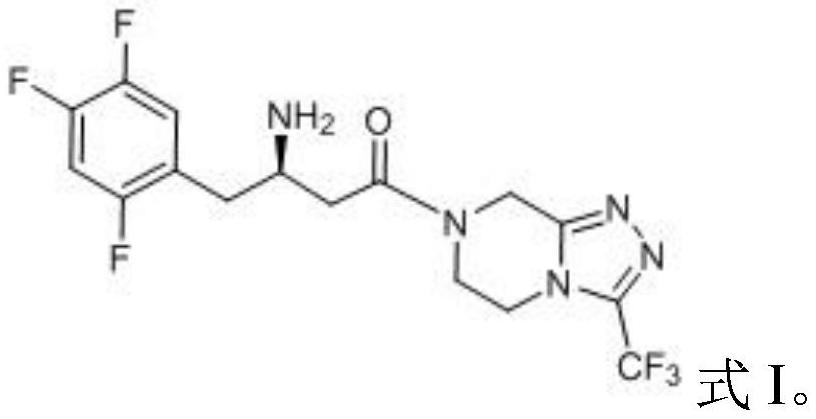
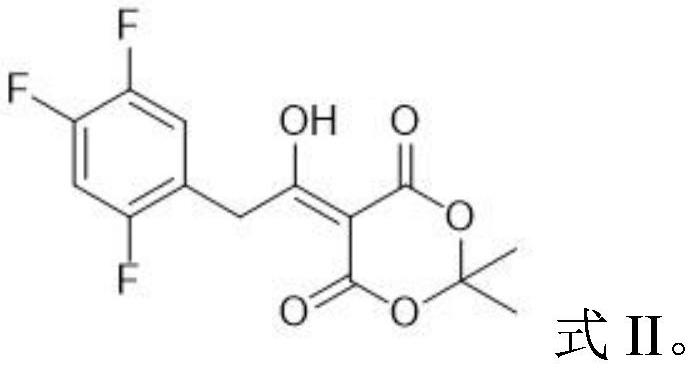
The invention relates to a synthesis method of a sitagliptin phosphate intermediate, and belongs to the technical field of organic synthesis. The method comprises the following technological steps of synthesizing a reaction liquid of 5-hydroxyl-[(2,4,5-trifluoro-phenyl)-ethylidene]-dimethyl-[1,3] dioxo-4,6-diketone from 2,4,5-trifluoro-phenylacetic acid, meldrum's acid, N,N-diisopropylethylamine, DMAP and acetylchloride; adding 3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4] triazol [4,3-alpha] pyrazine hydrochloride and trifluoroacetic acid to obtain the product. According to the method, the reaction raw materials are easily available; the reaction process is simple in operation; the requirements on reaction equipment are low; the reaction conditions are relatively mild; the yield and content are high, the quantity of waste water and solid wastes is relatively low, the cost is saved, and the method is suitable for industrial production.
Owner:SULI PHARMA TECH JIANGYIN
1,4-dihydropyridine derivatives as well as preparation method and application thereof
ActiveCN107382978AGreat research valueOrganic chemistry methodsForce measurement by measuring optical property variationChemical synthesisDihydropyridine
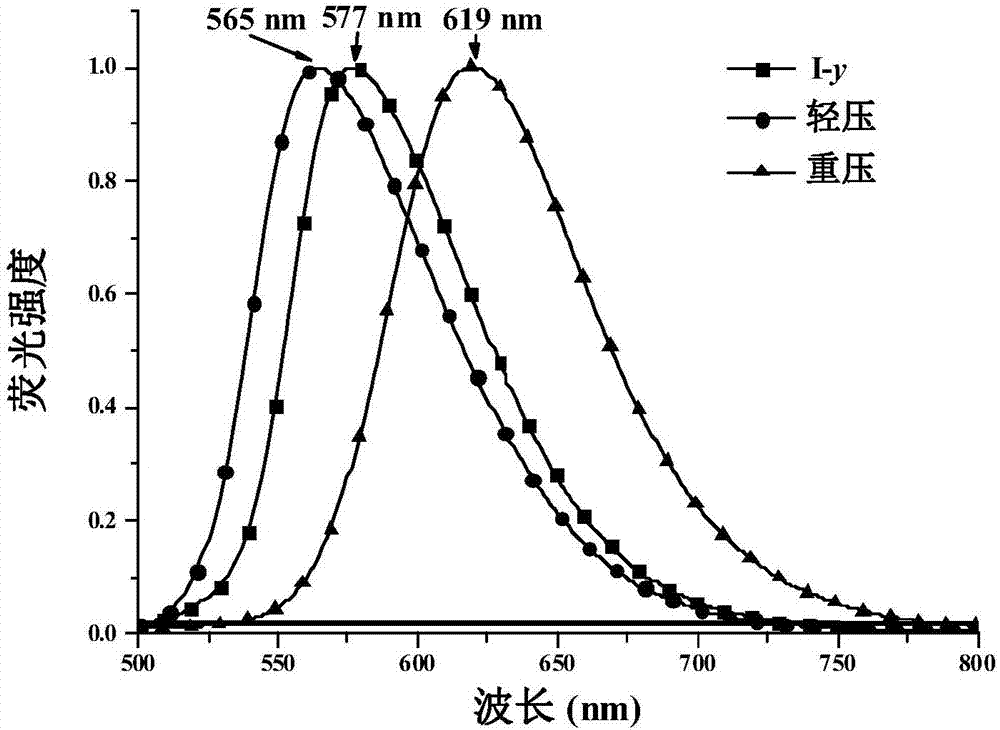
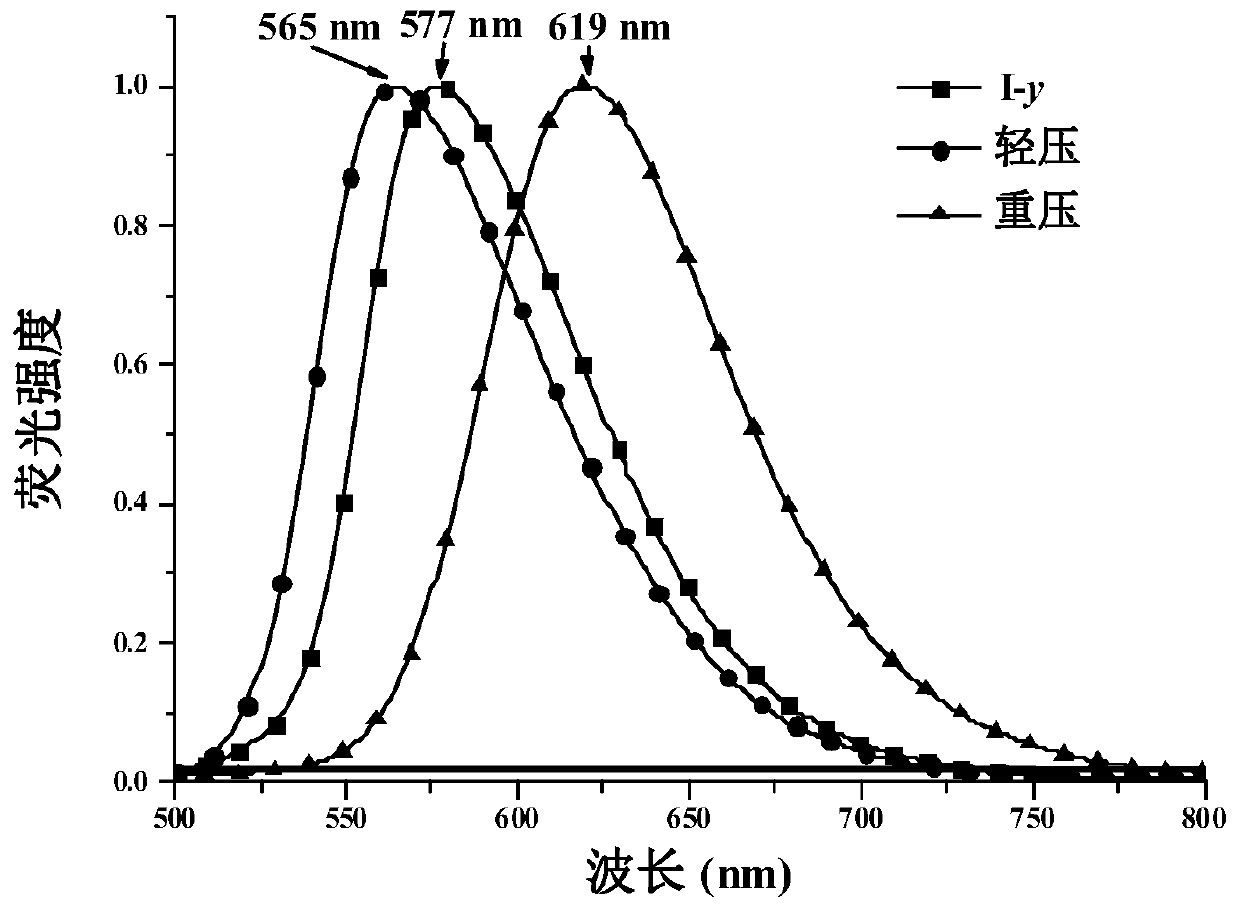
The invention belongs to the field of organic chemical synthesis and application and relates to 1,4-dihydropyridine derivatives as well as a preparation method and an application thereof. The preparation method of the 1,4-dihydropyridine derivatives comprises the following steps: (1) preparing an initial sample of the 1,4-dihydropyridine derivatives; (2) recrystallizing the initial sample of the 1,4-dihydropyridine derivatives in different ways to obtain three different crystalline compounds I-y, I-o and I-r, wherein the step (1) comprises the following steps: (11) forming an intermediate 2 from 2,6-dimethyl-4H-pyran-4-one 1 as an initial material as well as Meldrum's acid through an addition-elimination reaction; (12) forming an intermediate 3 from the intermediate 2 and 4-dimethylaminobenzaldehyde through a condensation reaction; (13) synthesizing the initial sample of the 1,4-dihydropyridine derivatives from the intermediate 3 and ethylamine through nucleophilic substitution.
Owner:WENZHOU UNIVERSITY
Photoresist resin monomer containing Meldrum's acid structure and synthesis method thereof
InactiveCN112661741AHigh-resolutionGood alkali solubilityOrganic chemistryPhotosensitive materials for photomechanical apparatusPolymer scienceImage resolution
The invention discloses a photoresist resin monomer containing a Meldrum's acid structure, and relates to the field of photoresist resin monomers. The structural formula of the photoresist resin monomer is shown in the specification, R1 is methyl or H, R2 is alkyl, naphthenic base, alkyl containing O atoms, or naphthenic base containing O atoms, R3 is H or alkyl, and the resin monomer is beneficial to increase of the alkali solubility of the photoresist resin, the edge side roughness of a photo-etched pattern can be improved, the etching resistance is better, the resolution of the photo-etched pattern is greatly improved, and the adsorption force of the photoresist on a silicon wafer can be improved.
Owner:上海博栋化学科技有限公司
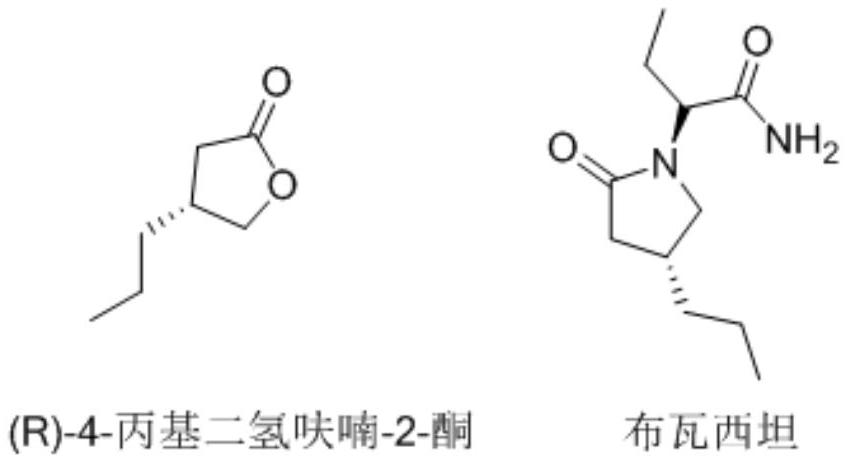
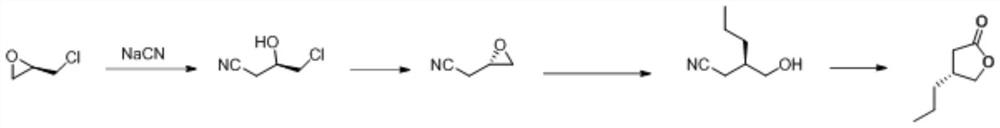
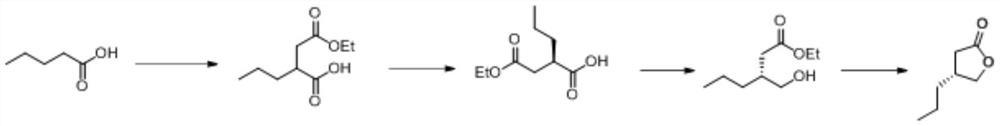
Synthesis method of (R)-4-propyldihydrofuran-2-ketone
ActiveCN112062740AThe route is short and novelHigh optical purityOrganic chemistry methodsChemical synthesisFuran
The invention discloses a synthesis method of (R)-4-propyldihydrofuran-2-ketone, and belongs to the field of organic chemical synthesis. A new process route is developed to overcome the defects that (R)-4-propyldihydrofuran-2-ketone is relatively high in material price, low in product purity, difficult to produce and amplify and the like. Meldrum's acid and R-epichlorohydrin are used as initial raw materials, and the compound is obtained by taking (1S, 5R)-3-oxabicyclo [3.1. 0]-2-hexanone as an intermediate through a two-step reaction of closing a three-membered ring and opening a ring by using a Grignard reagent. The (R)-4-propyldihydrofuran-2-ketone is synthesized by adopting the synthesis method, a novel process route is adopted in the process, the total molar yield is greater than 50%,and the method has the characteristics of novel and short route, high optical purity, low cost and the like.
Owner:SHENZHEN HUAXIAN PHARMA TECH CO LTD
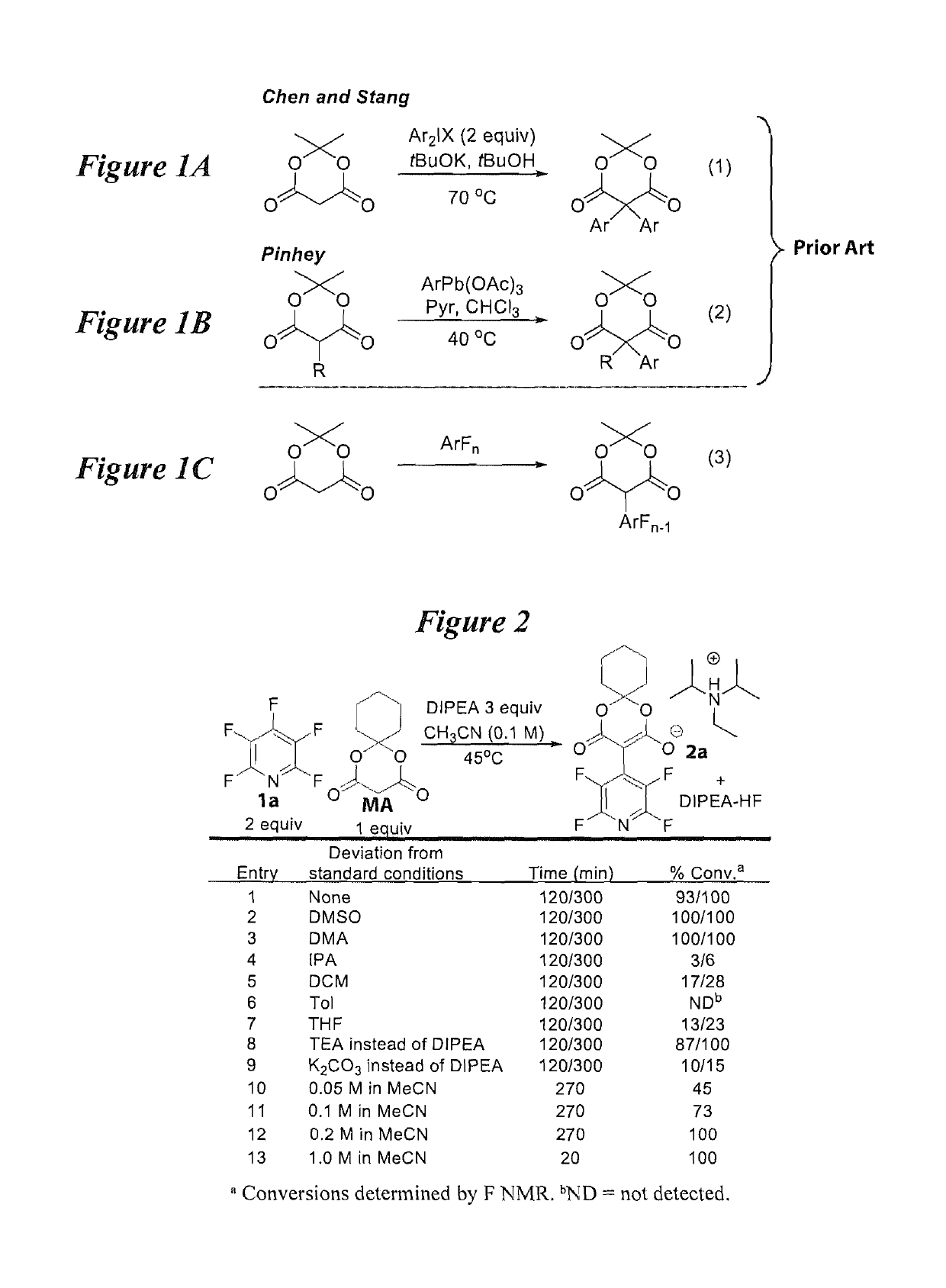
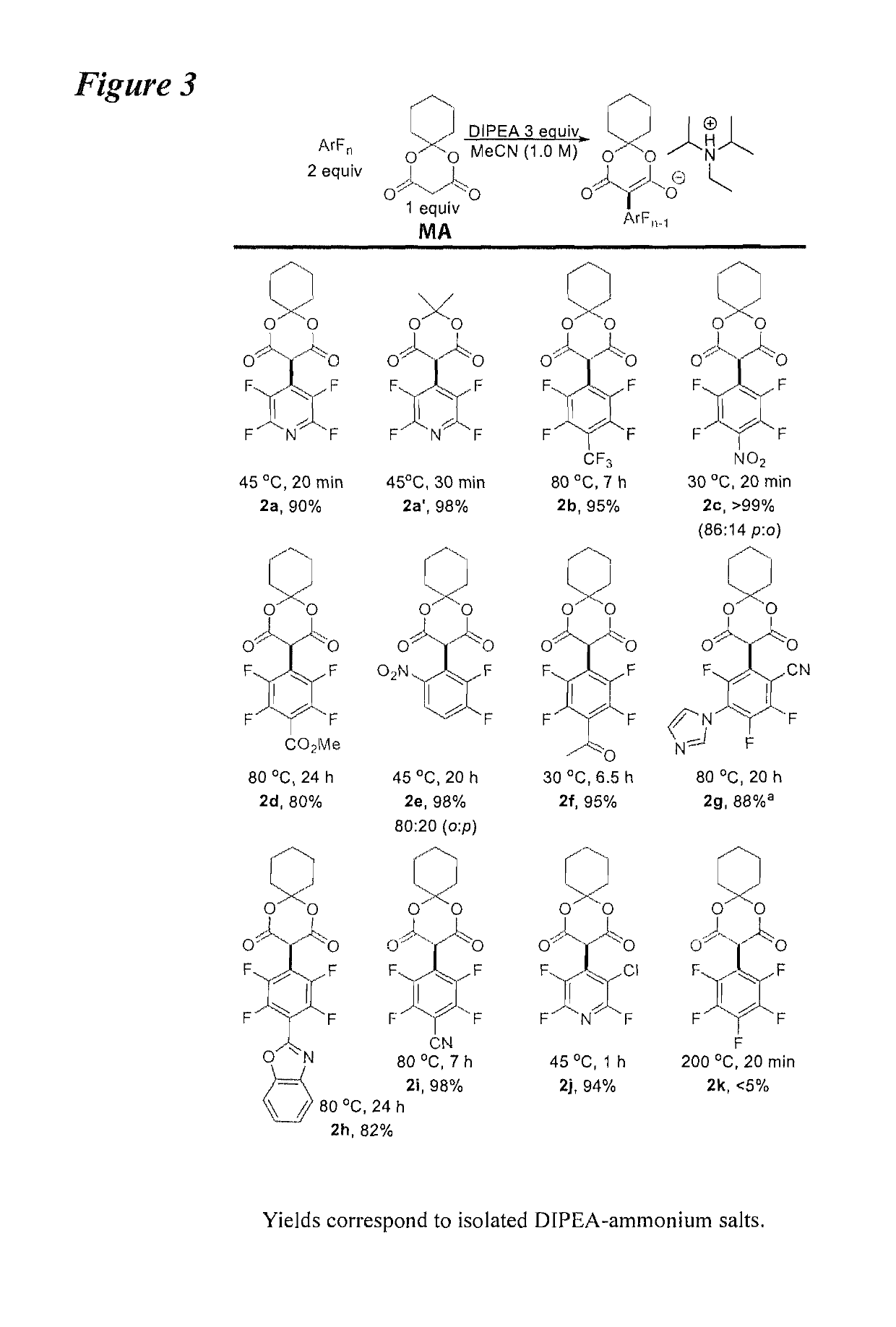
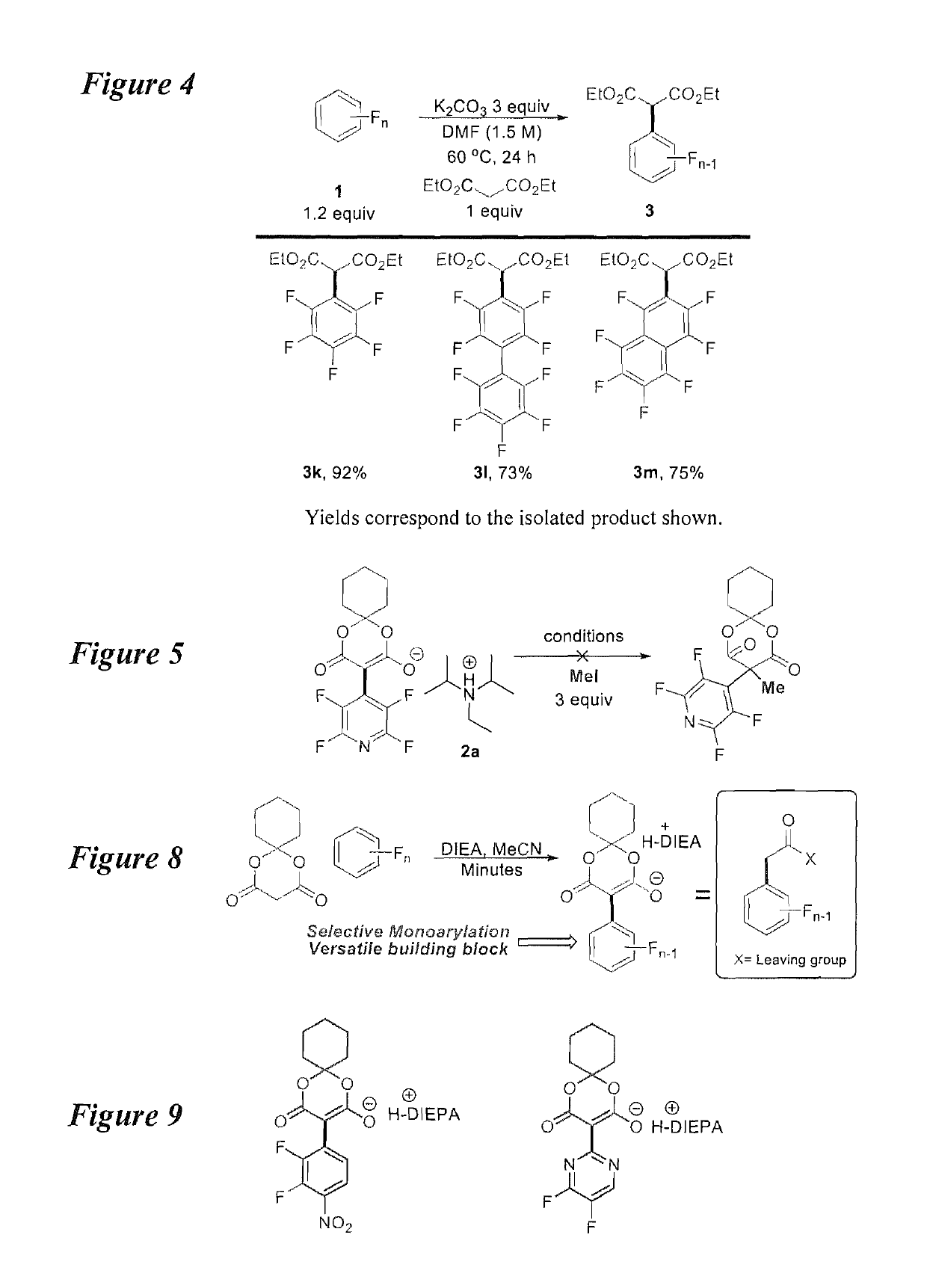
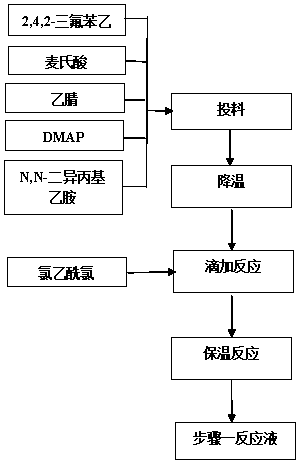
Facile and selective perfluoro-and polyfluoroarylation of meldrums acid
InactiveUS10316014B2Clean rapid operationally simple monoarylationEasy to functionalizeOrganic chemistryPhenyl acetic acidChromatographic separation
This disclosure relates generally to the facile and selective mono-perfluoro and -polyfluoroarylation of Meldrum's acid to generate a versatile synthon for highly fluorinated alpha-phenyl acetic acid derivatives which provide straightforward access to fluorinated building blocks. The reaction takes place quickly and all products were isolated without the need for chromatography. An embodiment provides an alternative strategy to access alpha-arylated Meldrum's acids which avoids the need for aryl-Pb(IV) salts or diaryliodonium salts and provides access to the tertiary product which was not previously synthetically accessible. The synthetic versatility and utility of the Meldrum's acid products is demonstrated by subjecting the products to several derivatizations of the Meldrum's acid products as well as photocatalytic hydrodefluorination which provide access to difficult but valuable synthetic targets such as multifluorinated aromatics.
Owner:BOARD OF REGENTS FOR OKLAHOMA STATE UNIVERSITY
A kind of synthetic method of sitagliptin phosphate intermediate
Owner:SULI PHARMA TECH JIANGYIN
Recycling method of acetyl Meldrum's acid derivative degradation waste
PendingCN113045448ARealize resource reuseReduce economic lossOrganic compound preparationCarboxylic acid esters preparationSitagliptinAcid derivative
The invention provides a recycling method of acetyl Meldrum's acid derivative degradation waste, and belongs to the technical field of resource reutilization. According to the method disclosed by the invention, acetyl Meldrum's acid derivative degradation waste is subjected to preheating treatment, alcoholysis reaction, ammonolysis reaction, acetylation reaction and purification treatment, the main components in theacetyl Meldrum's acid derivative degradation waste are converted, and a recovery product is finally obtained and can be used as an important intermediate in a sitagliptin production process, so that waste resource reutilization in the production process of the sitagliptin bulk drug is realized, economic loss caused by degradation of the acetyl Meldrum's acid derivative in the production process of the sitagliptin bulk drug is reduced, the problem of environmental pollution caused by degradation of the acetyl Meldrum's acid derivative in the production process of the sitagliptin bulk drug is eliminated, and the sustainable development of a sitagliptin raw material medicine green pharmaceutical process is promoted.
Owner:TAIZHOU BIOMEDICAL & CHEM IND RES INST CO LTD
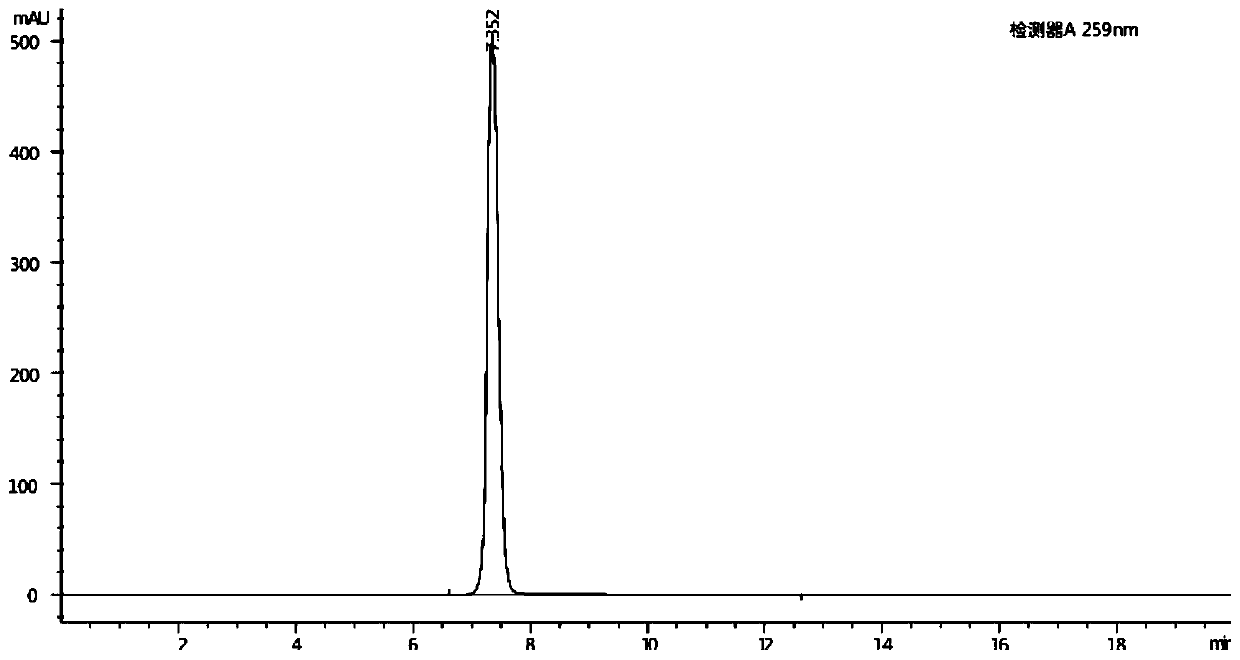
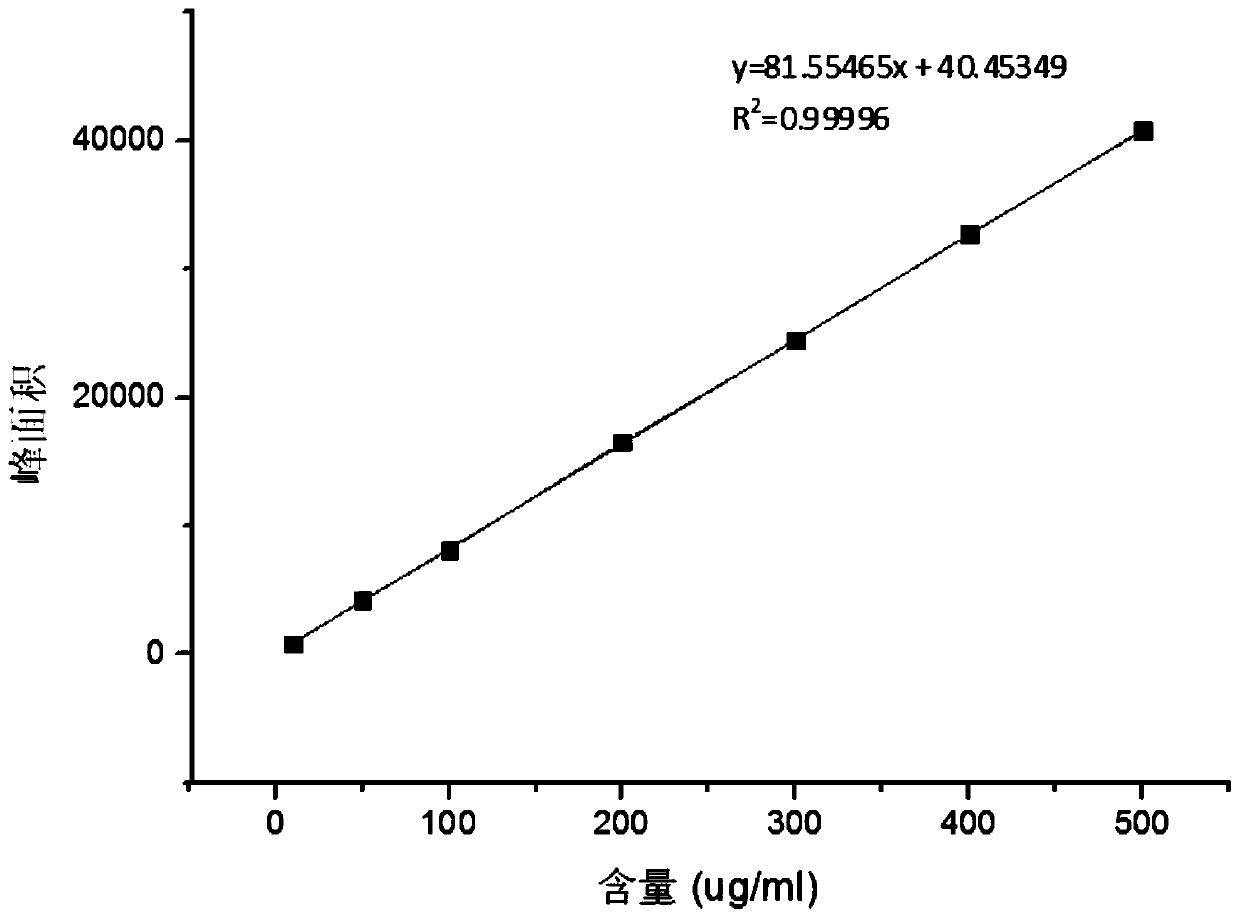
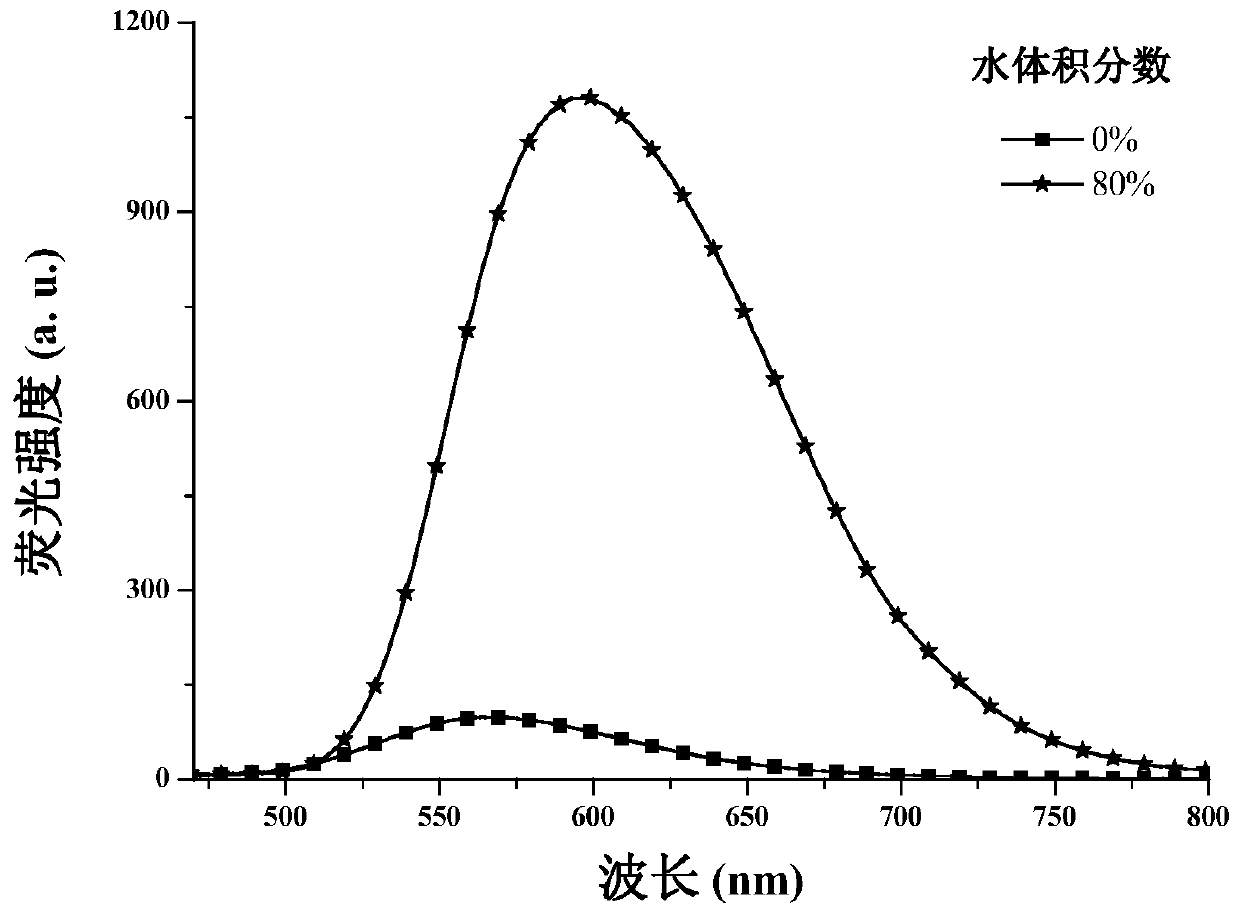
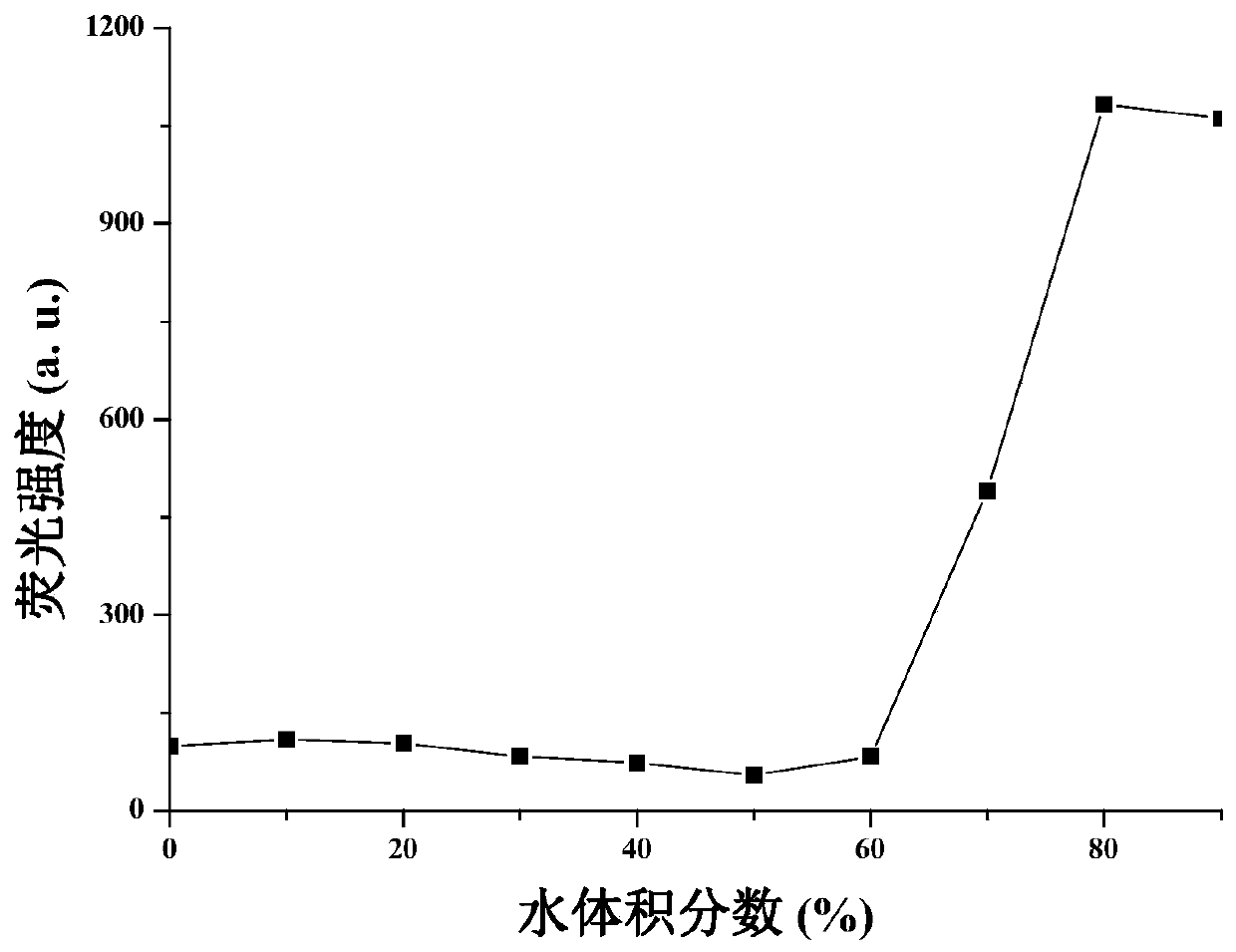
Method for measuring content of Meldrum's acid
The invention discloses a method for measuring the content of Meldrum's acid and is characterized in that peak areas of Meldrum's acid in a solution of a Meldrum's acid standard product and a solutionof a Meldrum's acid sample for test are separately measured by high performance liquid chromatography; and according to the obtained peak areas, calculating the mass content of Meldrum's acid in thesolution of the Meldrum's acid sample for test by using an external standard method. The method, which specifically limits chromatographic conditions for applying high performance liquid chromatography to measure the content of Meldrum's acid, is a simple content detection method with good separation effect, high accuracy and high precision. The method has a wide detection range and is accurate indetection results.
Owner:SHANGHAI JINGCHUN BIOCHEM TECH CO LTD
1,4-dihydropyridine derivatives and their preparation methods and uses
ActiveCN107382978BGreat research valueOrganic chemistry methodsForce measurement by measuring optical property variationChemical synthesisNucleophilic substitution
The invention belongs to the field of organic chemical synthesis and application and relates to 1,4-dihydropyridine derivatives as well as a preparation method and an application thereof. The preparation method of the 1,4-dihydropyridine derivatives comprises the following steps: (1) preparing an initial sample of the 1,4-dihydropyridine derivatives; (2) recrystallizing the initial sample of the 1,4-dihydropyridine derivatives in different ways to obtain three different crystalline compounds I-y, I-o and I-r, wherein the step (1) comprises the following steps: (11) forming an intermediate 2 from 2,6-dimethyl-4H-pyran-4-one 1 as an initial material as well as Meldrum's acid through an addition-elimination reaction; (12) forming an intermediate 3 from the intermediate 2 and 4-dimethylaminobenzaldehyde through a condensation reaction; (13) synthesizing the initial sample of the 1,4-dihydropyridine derivatives from the intermediate 3 and ethylamine through nucleophilic substitution.
Owner:WENZHOU UNIV
A kind of industrial preparation method of high optical purity acetyl tetrahydrofuran
The invention discloses an industrial preparation method of acetyl tetrahydrofuran with a high optical purity, and belongs to the field of chemical synthesis. According to the preparation method, tetrahydrofuroic acid is taken as the raw material and then is chlorinated to obtain tetrahydrofuran carbonyl chloride, tetrahydrofuran carbonyl chloride and Meldrum's acid carry out condensation reactions, and reaction product is hydrolyzed to obtain the target compound namely acetyl tetrahydrofuran. The preparation method has the advantages that the raw material cost is low, the preparation method does not need any Grignard reagent, the product property is stable, the purity can reach 98% or more, the optical purity can reach 99% or more, and the yield can reach 70% or more. The method has applied to industrial production. The product quality is stable. The reaction conditions are mild. The operation is safe and reliable. Dichloromethane can be recycled. The technology has the advantages of good repeatability and low preparation cost, and is a reliable industrial production method of acetyl tetrahydrofuran with a high optical purity.
Owner:CHENGDU LIKAI CHIRAL TECH
Anticancer 1,3-dioxane-4,6-dione derivatives and method of combinatorial synthesis thereof
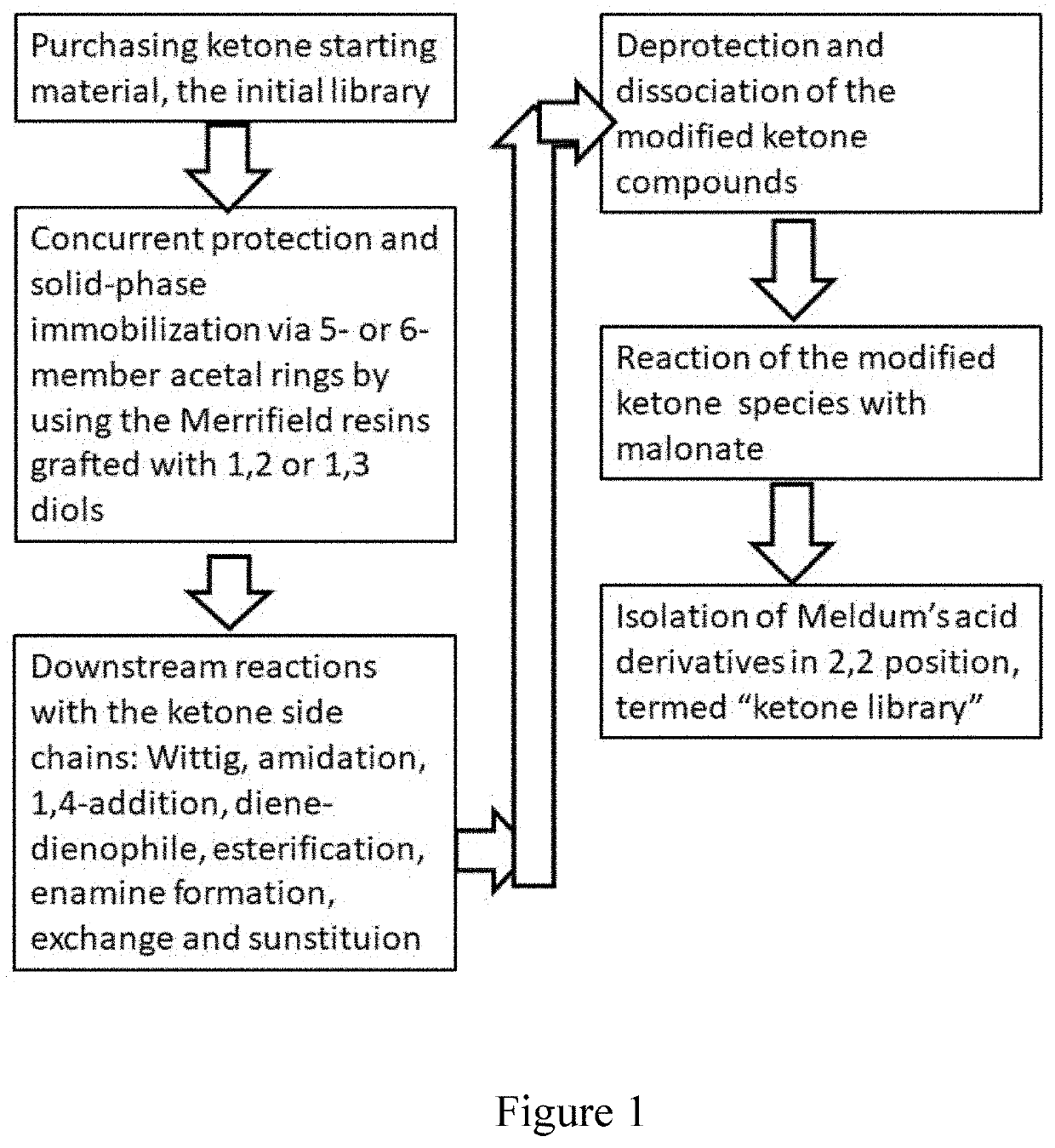
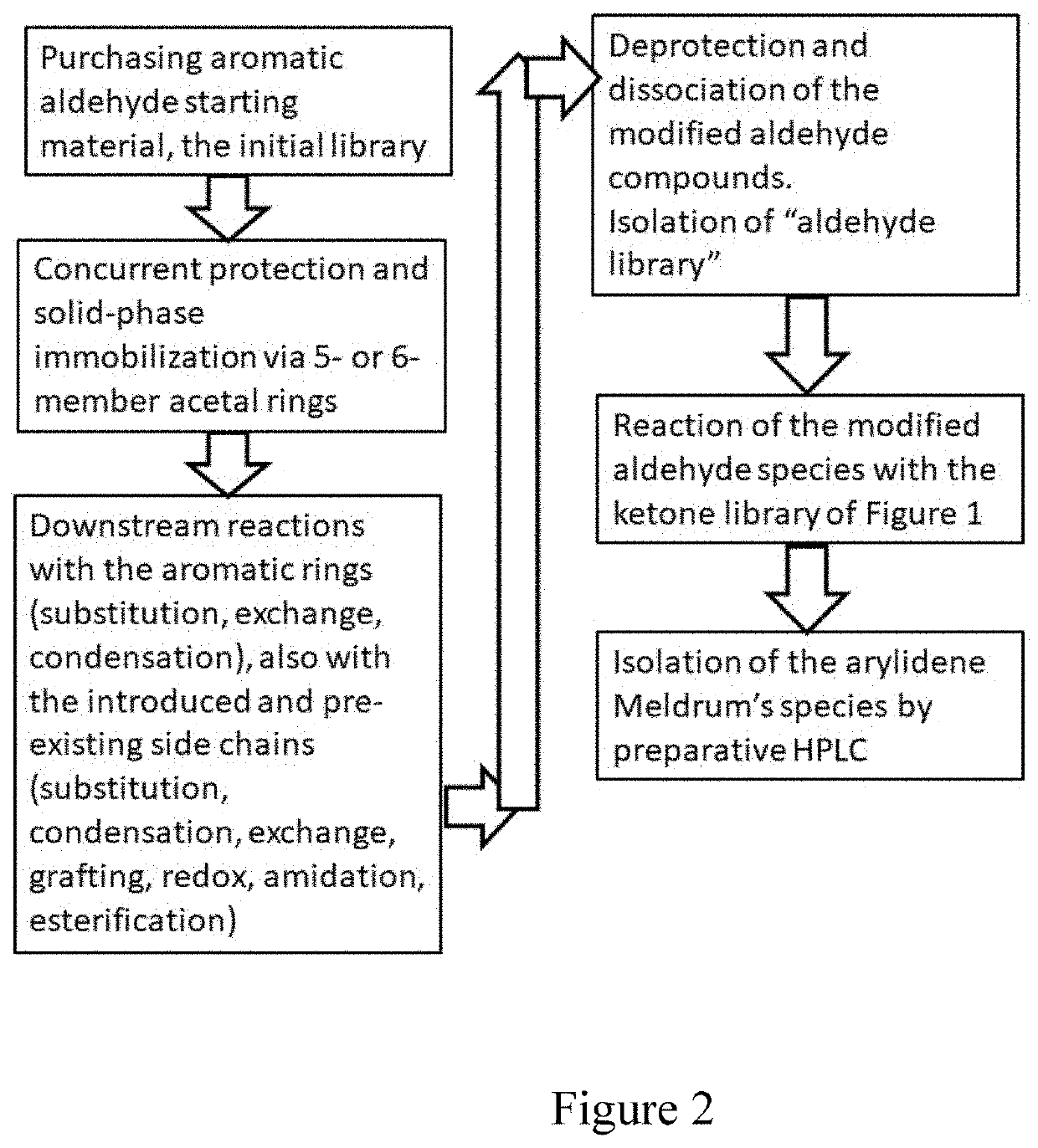
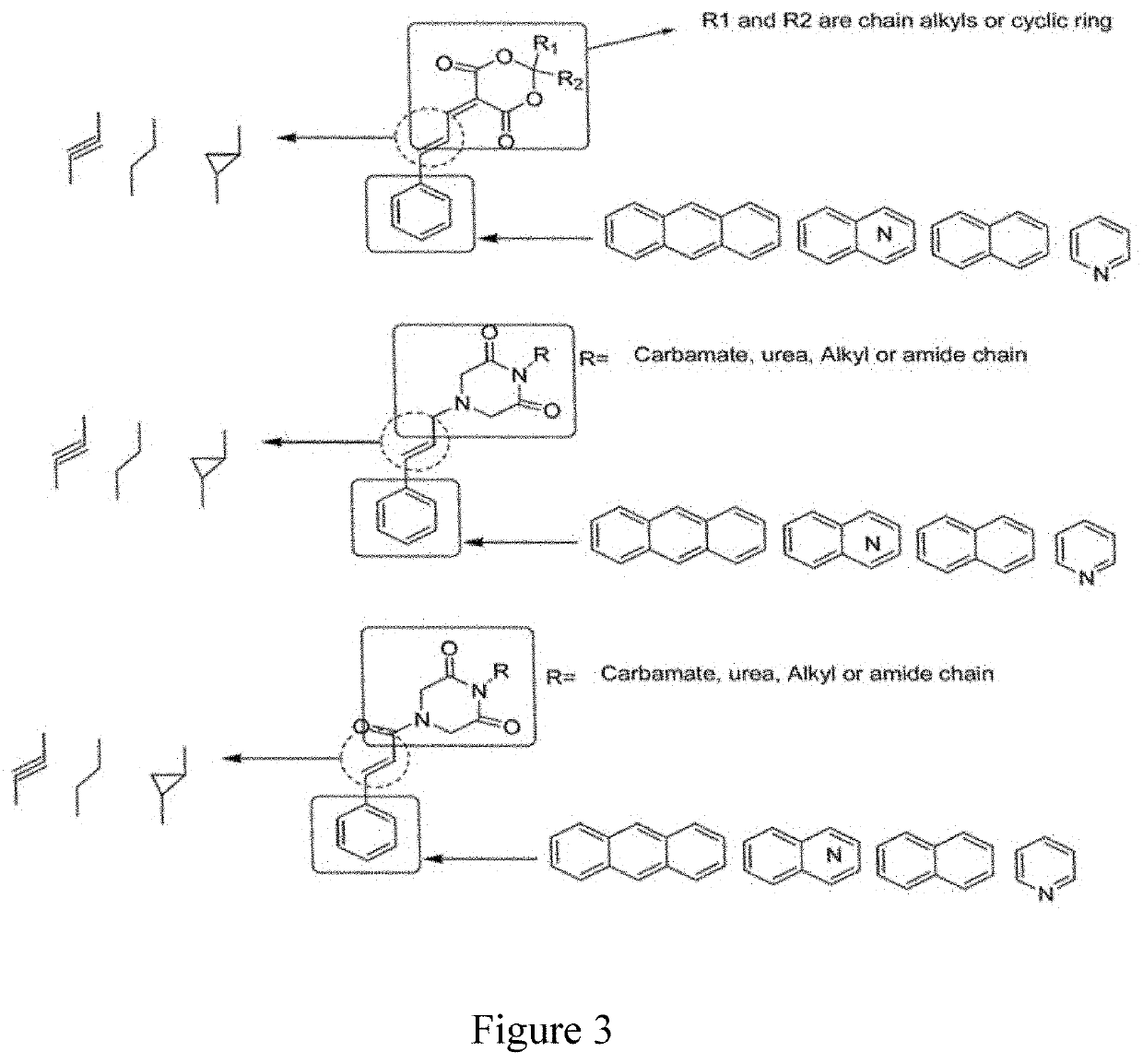
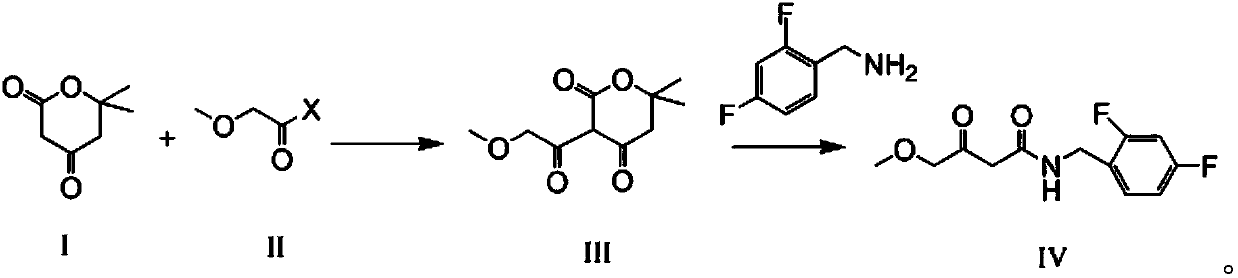
Compounds, methods of synthesis, and methods of cancer treatment by arylidene-1,3-dioxane-4,6-diones. A Meldrum's acid-based chemistry and hybrid solid-liquid method. The method includes protection of ketone and aldehyde components and simultaneous immobilization on the solid phase, introduction of substituents, grafts and derivatives compatible with the protection, detachment and restoration of active carbonyl reactivity, reaction of ketone library with malonate, reacting of the products with the aldehyde library in liquid phase and separation of the products by preparative HPLC.
Owner:NAT GUARD HEALTH AFFAIRS +2
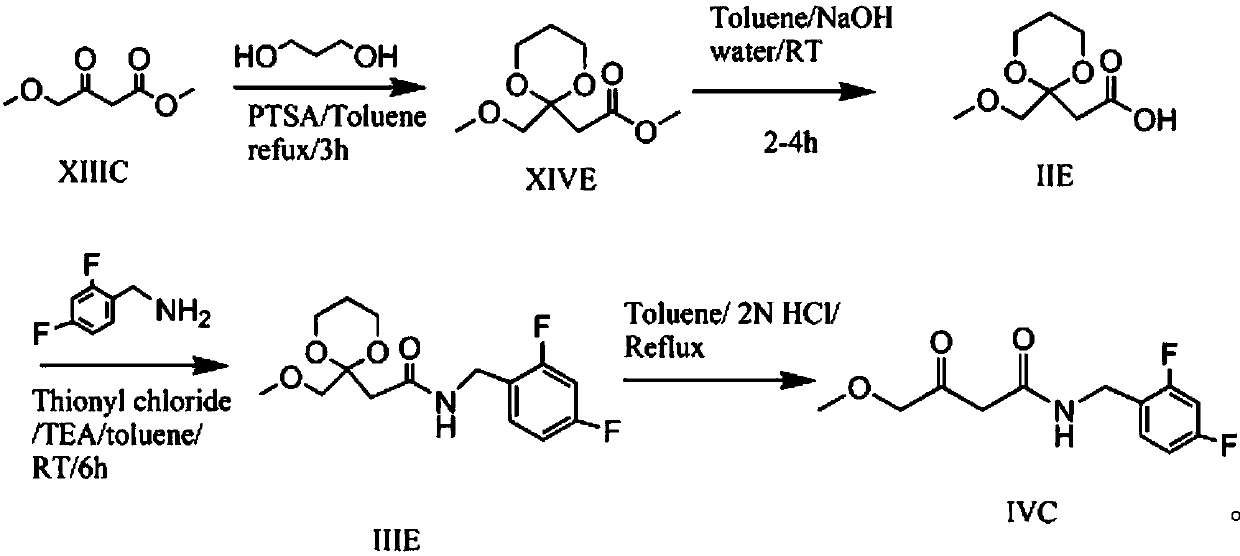
Preparation method of N-(2,4-fluorobenzoyl)-4-methoxyl-3-oxobutyrylamide
ActiveCN107935881ASimple and fast operationRaw materials are easy to getOrganic compound preparationCarboxylic acid amides preparationCombinatorial chemistryMeldrum's acid
The invention provides a preparation method of N-(2,4-fluorobenzoyl)-4-methoxyl-3-oxobutyrylamide. In the preparation method provided by the invention, Meldrum's acid is adopted as a raw material which is simple and easily available; moreover, the reaction process is short, the reaction conditions are mild, thus the preparation method is suitable for industrial and large-scale production of a Dolutegravir intermediate compound N-(2,4-fluorobenzoyl)-4-methoxyl-3-oxobutyrylamide.
Owner:ZHEJIANG LANGHUA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com