Preparation method for coumarin-3-carboxylic ester derivative
A technology for derivatives and coumarin, which is applied in the field of preparation of coumarin-3-carboxylate derivatives, can solve the problems of complicated steps, unenvironmental protection, and does not conform to the concept of green chemistry, and achieves simple steps and high catalytic efficiency. small amount of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention provides a method for preparing coumarin-3-carboxylate derivatives as shown in formula (I) or formula (II), wherein the method comprises: 3 In the presence of , the salicylaldehyde derivative shown in formula (III) or 2-hydroxyl-1-naphthaldehyde shown in formula (IV) and the general formula are R 2 OH alcohol and Michaelis acid contact reaction,
[0033]
[0034] Among them, R 2 for C 1 -C 8 Alkyl or C 7 -C 13 The aryl; n is 1-4; R 1 selected from H, halogen, nitro, C 1 -C 6 Alkoxyl, C 1 -C 10 of alkyl and amine groups.
[0035] In the preparation method, when n in the salicylaldehyde derivative shown in formula (III) is 1-4, regardless of R 1 Whether it is a strong electron-withdrawing group substitution (such as nitro, fluorine, etc.) or a strong electron-donating group substitution (such as methoxy, diethylamino, etc.) is applicable to this method. In the case of n=2-4, R 1 Preferred is 3,5-di-tert-butyl.
[0036] R in formula (...
Embodiment 1-1
[0045] Synthesis of coumarin-3-methyl carboxylate: weigh 0.366g (3mmol) of salicylaldehyde, 0.518g (3.6mmol) of Michaelis acid, 5mL of methanol and 0.0082g (0.05mmol) of ferric chloride In the bottom flask, heated to 70°C and refluxed in an oil bath with stirring for 6h. After the reaction was completed, cooled to room temperature, concentrated the reaction solution, and then separated by column chromatography to obtain 0.538g of white solid product with a yield of 88%.
[0046]
[0047] The structure, color, state, yield and characterization data of coumarin-3-methyl carboxylate are as follows:
[0048]
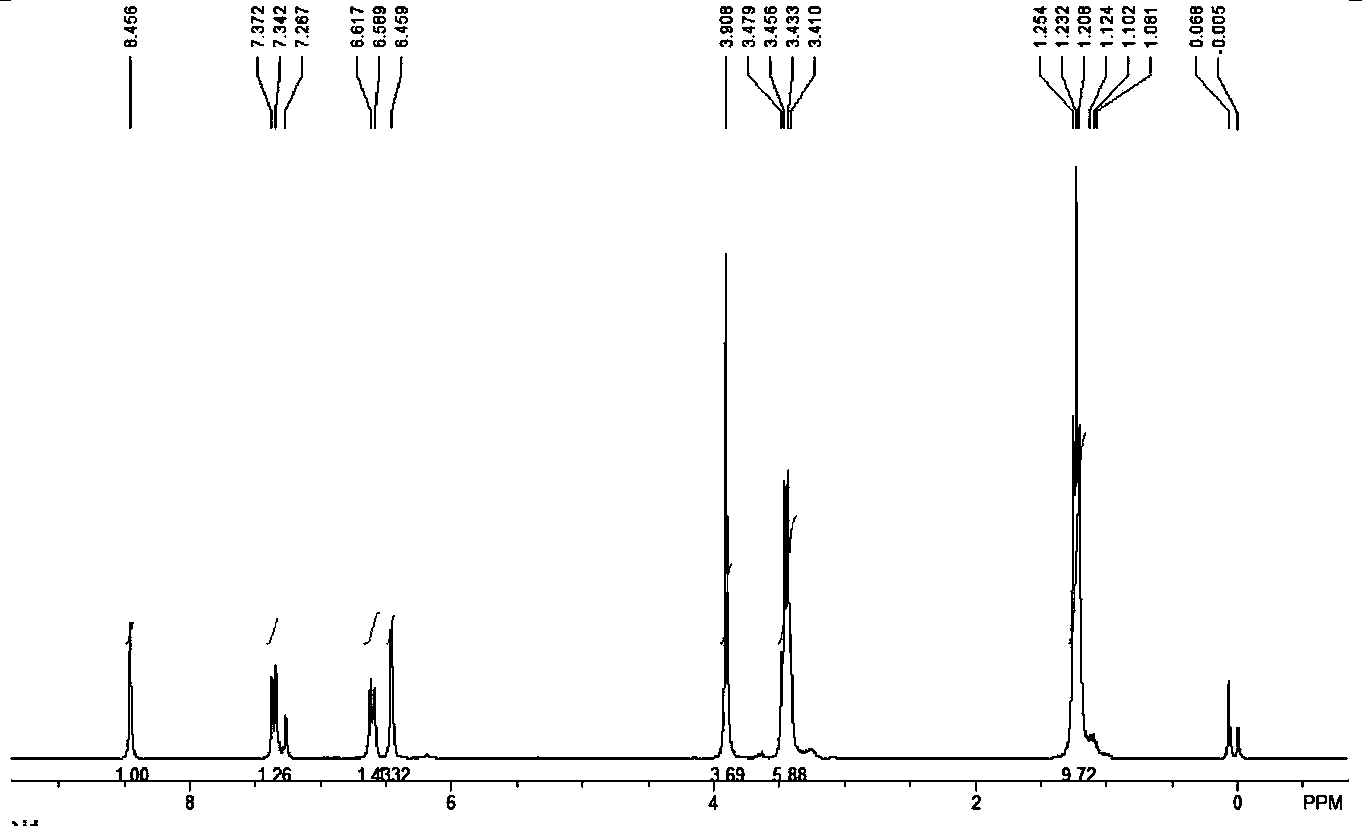
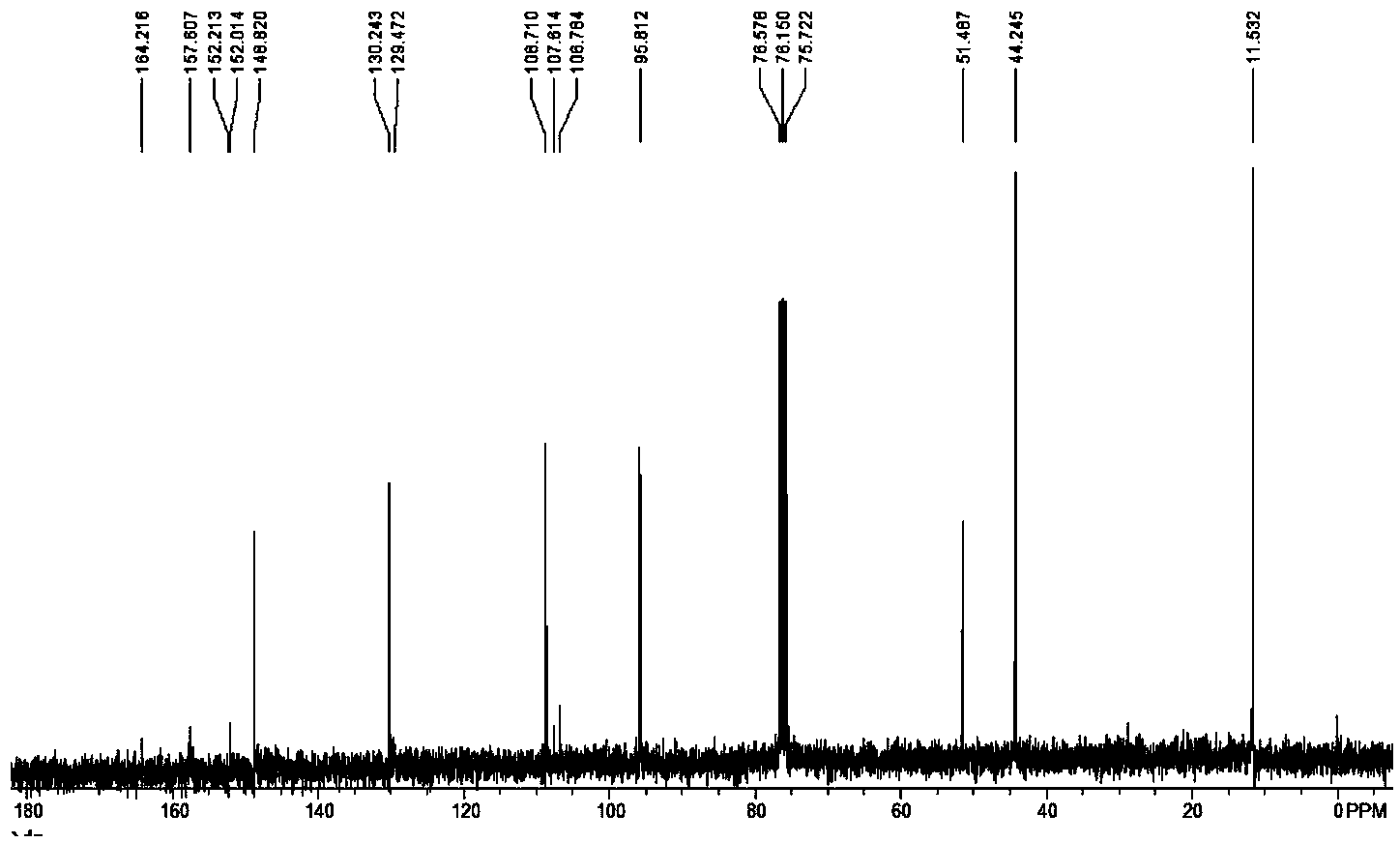
[0049] Methyl coumarin-3-carboxylate: white solid; yield 88%; 1 HNMR (300MHz, CDCl 3 ) δ: 8.53 (s, 1H, CH), 7.56-7.64 (m, 2H, ArH), 7.28-7.34 (m, 2H, ArH), 3.92 (s, 3H, CH 3 ) ppm; 3 CNMR (75MHz, CDCl 3 ) δ: 163.7, 155.2, 149.1, 134.4, 129.5, 124.9, 117.9, 117.8, 116.7, 52.9ppm.
Embodiment 1-2
[0051] Synthesis of 6-chlorocoumarin-3-methyl carboxylate: according to the preparation method of Example 1-1, the difference is that 3 mmol of the salicylaldehyde derivative shown in formula (A) is used instead of Example 1-1 Salicylaldehyde in.
[0052]
[0053] The structure, color, state, yield and characterization data of 6-chlorocoumarin-3-methyl carboxylate are as follows:
[0054]
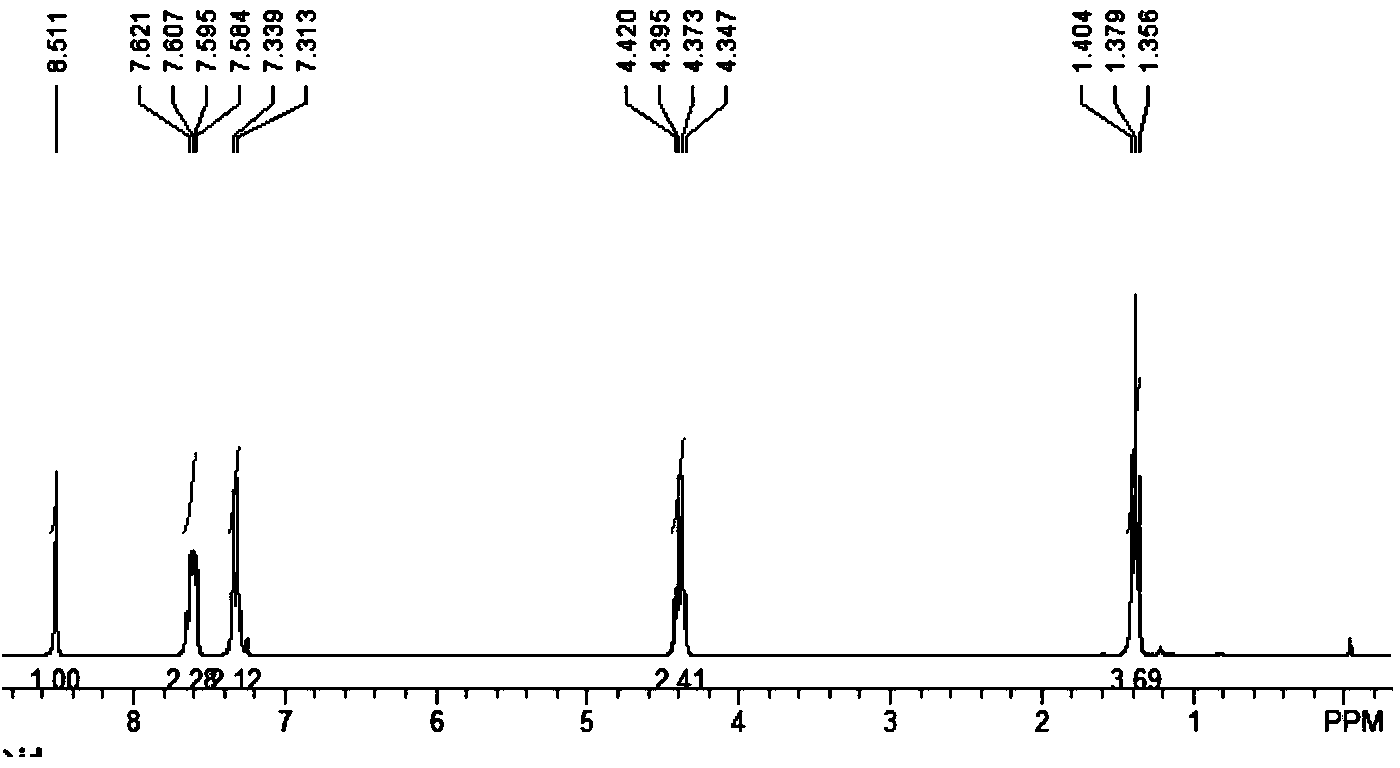
[0055] Methyl 6-chlorocoumarin-3-carboxylate: white solid; yield 83%; 1 HNMR (300MHz, CDCl 3 ) δ: 8.43 (s, 1H, CH), 7.70 (d, J=8.7Hz, 1H, ArH), 7.55 (d, J=8.7Hz, 1H, ArH), 7.21 (s, 1H, ArH), 3.92 (s, 3H, CH 3 ) ppm; 13 CNMR (75MHz, CDCl 3 ) δ: 163.4, 161.8, 150.1, 147.7, 135.6, 134.3, 133.2, 131.8, 130.2, 129.3, 119.3, 118.6, 53.0ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com