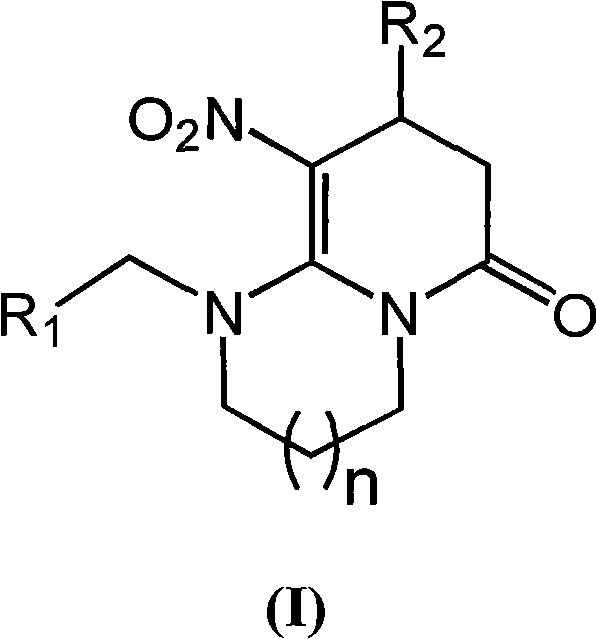
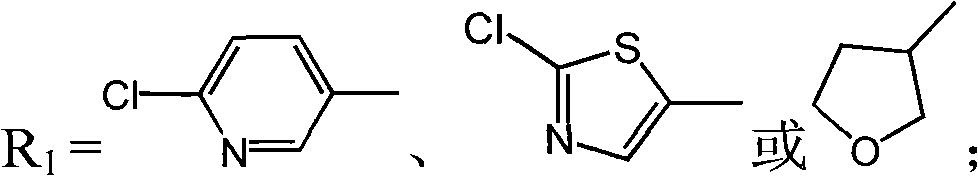
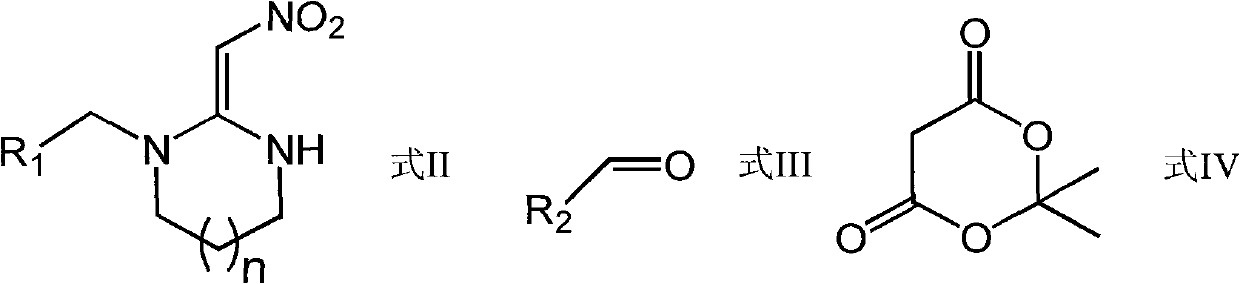
Tetrahydropyridone 1,3-diazacyclo cis-neonicotine compounds as well as preparation method and applications thereof
A technology of tetrahydropyridone and diazine heterocycles, which is applied in the field of pesticides and insecticides, can solve problems such as restricting development and limiting applications, and achieve high-efficiency insecticidal activity, simple preparation process, and safety for humans and animals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of cis-1-(6-chloro-3-pyridylmethyl)-7-phenyl-8-nitro-1,2,3,5,6,7-hexahydroimidazol[1,2-a] Pyridin-5-one;
[0041] The raw material 2-chloro-5-(2-nitromethylene-1-imidazolylmethyl)-pyridine was synthesized according to the literature method: (a) Zhong Li; Wenwen Zhang; Zewen Liu; J.Agric.Food. Chem.2010.58.6296-6299; (b) Xusheng Shao, Zhong Li, XuHong, Qian, and Xiaoyong Xu.J.Agric.Food.Chem.2009.57.951-957.
[0042] The specific steps are:
[0043]
[0044] (1) Synthesis of N'-(6-chloro-3 pyridylmethyl)-1,2-ethylenediamine:
[0045] Get 16.1g (100mmol) 2-chloro-5-chloromethylpyridine and dissolve it in 100ml acetonitrile, and slowly add it dropwise to the mixed solution composed of 30.30g (500mmol) ethylenediamine and 80ml acetonitrile under the condition of ice bath, Adding time is two and a half hours. After continuing the reaction for 8 hours, the reaction was stopped, and part of the acetonitrile was removed by rotary evaporation. Add 100ml of wa...
Embodiment 2
[0058] Preparation of cis-1-(6-chloro-3-pyridylmethyl)-7-(4-chlorophenyl)-8-nitro-1,2,3,5,6,7-hexahydroimidazolium[ 1,2-a]pyridin-5-one.
[0059] The benzaldehyde in Example 1 was replaced by 4-chlorobenzaldehyde, and the other conditions were the same as in Example 1 to obtain a yellow solid with a yield of 90.2%.
[0060] Elemental analysis: found value C% 54.40 H% 3.85 N% 13.35
[0061] Calculated C% 54.43 H% 3.85 N% 13.36
[0062] IR (KBr, cm -1 )v max 2978, 2359, 1685, 1650, 1481, 1423, 1418, 1257, 1226, 1095, 892.
[0063] 1 H NMR (CDCl 3 , 400MHz) δ8.29(s, 1H, Pyridine), 7.51(dd, J=2.5, 8.5Hz, 1H), 7.22-7.28(m, 2H), 7.22(d, J=8.2Hz, 1H), 7.07 -7.13(m, 2H), 4.89(t, J=4.4Hz, 1H), 4.95(d, J=3.9Hz, 2H), 3.64-4.46(m, 4H), 3.12(d, J=4.5Hz, 2H).
[0064] The product prepared in this embodiment is made into an aqueous suspension, sprayed with water, and has a killing rate of more than 95% against spider mite, has high-efficiency insecticidal activity, and is...
Embodiment 3
[0066] Preparation of cis-1-(6-chloro-3-pyridylmethyl)-7-(3-chlorophenyl)-8-nitro-1,2,3,5,6,7-hexahydroimidazol[1 , 2-a] pyridin-5-one.
[0067] The benzaldehyde in Example 1 was replaced by m-chlorobenzaldehyde, and other conditions were the same as in Example 1 to obtain a yellow solid with a yield of 83.5%.
[0068] Elemental analysis: found value C% 54.41 H% 3.82 N% 13.33
[0069] Calculated C% 54.43 H% 3.85 N% 13.36
[0070] IR (KBr, cm -1 )v max 2998, 2367, 1783, 1648, 1485, 1313, 1248, 1227, 1216, 1007, 880.
[0071] 1 H NMR (CDCl 3 , 400MHz) δ8.20(s, 1H, Pyridine), 7.28-7.38(m, 3H), 7.20(d, J=8.2Hz, 1H), 7.08-7.16(m, 2H), 4.97(t, J= 4.3Hz, 1H), 4.79(d, J=3.9Hz, 2H), 3.72-4.45(m, 4H), 3.10(d, J=4.5Hz, 2H).
[0072] The product prepared in this embodiment is made into water-emulsion insecticide, mixed with water and sprayed, and the killing rate to rice planthopper is more than 90%, has high-efficiency insecticidal activity, and is safe for humans and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com