Preparation method of 5-( arylmethylene) meldrum's acid
An arylmethylene and arylmethylene aniline technology, which is applied in the field of preparation of 5-Midde acid, can solve the problems of high environmental harm, long reaction time, strong human toxicity, etc., and achieves environmental friendliness, convenient post-processing, The effect of high product yield
Inactive Publication Date: 2010-12-22
SHANGHAI UNIV
View PDF1 Cites 6 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In this way, it cannot continue to carry out the cyclization reaction as a Michael addition reaction acceptor;
Secondly, the solvent used in the method provided in Document 2 is anhydrous benzene. It is known that benzene belongs to the first class of solvents in the ICH classification of chemical solvent toxicity, that is, it is a substance with strong toxicity to humans and relatively harmful to the environment;
The 3rd, the method that document 2 provides adopts pyrazole acetate as catalyst, and the reaction time is longer
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
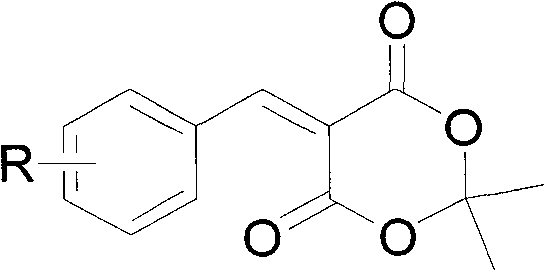
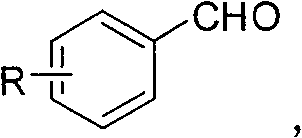
The invention relates to a preparation method of 5-(arylmethylene) meldrum's acid, which comprises the following steps of: a, dissolving aromatic aldehyde and aniline according to the mol rate of 1.0:(1.0-1.2) into water, wherein the aromatic aldehyde has a structural formula described in the specification, R is H, X (halogen), alkyl (such as CH3, C2H5), alkoxy (such as OCH3, OCH2CH3), OH, NO2 and CN; and b, respectively dissolving arylmethylene aniline obtained from the step a and meldrum's acid into an alcohol solution according to the mol ratio of (1.1-1.2):1.0 to obtain respective saturated solutions, then adding the alcohol saturated solution of the arylmethylene aniline into the alcohol saturated solution of the meldrum's acid, stirring for reacting for 3-4min, filtering after the reaction is ended, washing filter residue with water, drying and then recrystallizing to obtain the 5-(arylmethylene) meldrum's acid. The preparation method has the advantages of environment protection because of using water and alcohol as solvents, mild reaction condition, convenient posttreatment and higher yield of products.
Description
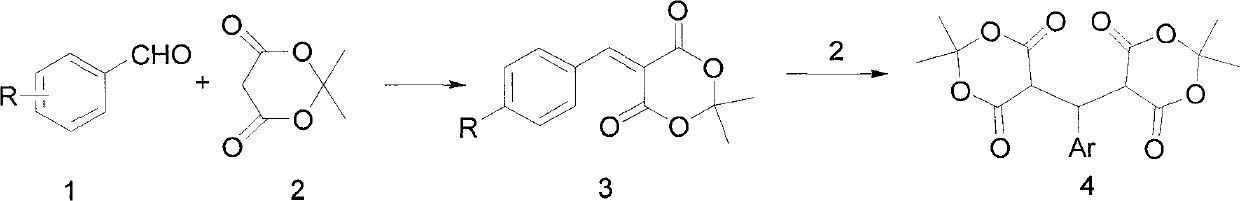
technical field The invention relates to a preparation method of 5-(aryl methylene) Michaelis acid. Background technique 5-(Arylene) Michaelis acid is a very active Michael addition reaction acceptor, which can be used as a reaction intermediate and a nucleophile to synthesize a series of heterogeneous compounds with potential medical value through D-A reaction and hetero D-A reaction. ring compound. Its English name is 5-Arylidene-2, 2-dimethyl-1, 3-dioxane-4, 6-diones (arylidene Meldrum's acids, AMAs). The chemical structural formula of 5-(aryl methylene) Michaelis acid is as follows: Wherein R=H, X (halogen), alkyl (such as: CH 3 , C 2 h 5 ), alkoxy (such as: OCH 3 , OCH 2 CH 3 ), OH, NO 2 , CN The synthetic route of 5-(arylmethylene) Michaelis acid reported in Document 1 (Tetrahedron Letters 42 2001 5203-5205) is shown in Scheme 1: using aromatic aldehyde 1 and Michaelis acid 2 as raw materials, using water as solvent in Knoevenagel condensation reaction w...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D319/06
Inventor 宋力平王鹏远戴柏凡易海王玮刘建宁张敏黄培刚
Owner SHANGHAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com